INITIAL PRESENTATION
Chief Complaint: Alkaline injury to right eye
History of Present Illness:
52-year-old male who presented emergently after an alkali chemical injury to his eyes. A dishwasher line exploded causing industrial grade dishwasher to splash directly onto his face. He reported instant vision loss in his right eye, and severe bilateral eye pain.
When initially evaluated at an outside hospital, the reported pH was 9.0 in his right eye and 8.5 in his left eye. An open globe was ruled out and he was irrigated with 2L normal saline in both eyes during transfer.
Past Ocular History:
Past Ocular Surgery/Laser:
Past Ocular Trauma:
Ocular Medications:
Past Medical History:
Medications:
Allergies: No known Allergies
Family History: The patient’s father has multiple sclerosis with speech and mobility limitations. No family ocular problems.
Social History:
Review of Systems: Negative except as stated in HPI
OCULAR EXAMINATION
Differential Diagnosis:
CLINICAL COURSE
In the ED, his initial pH was 9.0 in the right eye and 8.5 in the left eye. He was irrigated with an additional 9-10L of normal saline, and a final pH of 7.5 was achieved bilaterally. He was started on a regimen of topical eye drops including prednisolone acetate 1% every 2 hours, ascorbic acid 10% every 2 hours, sodium citrate 10% every 2 hours, medroxyprogesterone 1% every 2 hours, moxifloxacin 0.5% 4 times daily, erythromycin ointment every night at bedtime, and artificial tears as needed in the right eye. In addition, he was prescribed oral doxycycline 100 mg two times a day and oral vitamin C 2 gram two times a day. At his follow-up visit two days later, the intraocular pressure in his right eye was 59 mm Hg, and his visual acuity in the right eye diminished to light perception. Repeat pH was 7.5 and additional irrigation was initiated in clinic with a final pH of 7.0. Cosopt, brimonidine, and oral acetazolamide were also given in clinic and his IOP OD improved to 36 mm Hg. B-scan ultrasound was repeated and did not show any evidence of retinal detachment. He was admitted for ocular surface reconstruction with amniotic membrane graft placement and necrotic tissue removal in the OR.
In the OR, the choice was made to forgo necrotic tissue debridement due to the large amount of damaged tissue and proceed with ocular surface reconstruction with amniotic membrane and Prokera ring placement only. At his postoperative visit, his amniotic membrane was found to be loosening and was removed, but the Prokera remained intact. The patient experienced significant retrobulbar pain that was only partially relieved with proparacaine. His visual acuity in his right eye improved to hand motions, and IOP remained stable in the high 20s. Unfortunately, at his one month postoperative visit, removal of the Prokera membrane revealed severe opacification of the superior limbus, cornea edema with Descemet folds superiorly, a circular corneal opacity superonasally, total epithelial defect from limbal stem cell deficiency, dehiscence of Descemet membrane, severe cicatricial trichiasis, and new hypopyon (Figure 2). Due to his severe pain, guarded visual prognosis, and concern for a new fungal keratitis, the patient elected to proceed with enucleation one month following his initial accident.
DIAGNOSIS: Chemical Eye Injury
DISCUSSION
Introduction
Chemical injuries to the eye are ophthalmic emergencies that require immediate management. Delay in care can result in deeper penetration of the chemical agent resulting in more widespread injury. Long term sequelae of chemical ocular burns include secondary glaucoma, limbal stem cell deficiency, and permanent vision loss [1].
Etiology/Epidemiology
Chemical ocular burns are a common cause of ocular trauma and are costly to both the patient and society at large [2]. From 2013 to 2016, 144,149 cases of chemical ocular burns were reported at emergency departments in the United States, leading to an estimated total of emergency department charges alone of approximately 26.6 million per year [3]. The Occupational Safety and Health Administration (OSHA) estimated that workplace eye injuries cost an estimated $300 million per year when accounting for lost productivity, medical treatment, and worker compensation [4].
Unlike many other ocular pathologies, chemical ocular burns tend to occur most commonly in younger age groups. One study found that the median patient age is 32 years old but that children between the ages 1 to 2 have a relatively high incidence of chemical eye injuries among the pediatric population [3]. More than two thirds of these injuries occur in the workplace setting, although residential exposure to chemicals is also common [5]. Males constitute more than 81% of workplace related eye injuries, while children are more likely to suffer from exposure to household chemicals such as topical personal agents [3] and laundry detergent pods [6]. Nonaccidental trauma from deliberate chemical assault attacks are not uncommon, and have been reported in many countries, with the most common chemical agent involved being sulfuric acid [7 ,8]. Chemical facial peeling agents, such as 35% trichloroacetic acid, have also been rarely reported to cause chemical ocular burns [9]. Understanding the demographics and settings in which chemical ocular burns occur is essential to developing better prevention strategies in the future.
Pathophysiology
Chemical eye injuries can be divided into alkaline (pH > 7.0), acidic (pH < 7.0), and neutral etiologies. Alkali injuries are more common than acidic injuries due to their ubiquity in common industrial and household cleaning reagents.
Alkali injuries are not only more common than acidic injuries in general, they also tend to lead to more severe ocular injury [10]. Alkali agents saponify the fatty acid components of cell membranes, which leads to rapid cell necrosis and allows for deeper penetration into the eye15. Additionally, the cation component of the alkali agent reacts with the carboxyl group of stromal collagen and glycosaminoglycans, which leads to opacification of the corneal stroma, distortion of the trabecular meshwork, and secondary spiking of intraocular pressure. Depending on the depth of penetration into the eye, other ocular structures may also be affected. Due to its rapid penetration, alkali injuries can take longer to neutralize the pH, with studies showing that anywhere from 30 minutes to 3 hours are required [11]. Of the alkali reagents, ammonia (NH3) and sodium hydroxide (NaOH), a common ingredient in lye, penetrate the most rapidly. Studies have shown that ammonia requires as little as 15 seconds to penetrate into the anterior chamber while sodium hydroxide takes about 3-5 minutes [12].
On the other hand, acidic agents are associated with milder ocular injury [10]. This is because the anion in acidic agents causes protein precipitation and denaturation in the corneal epithelium and superficial stroma, which then acts as a barrier to prevent deeper penetration of the H+ cation into the eye. The important exceptions to this are hydrofluoric acid and, to a lesser extent, sulfurous acid (H2SO3), both of which can penetrate rapidly into the eye due to their small size and low molecular weight. Sulfuric acid (H2SO4), the most common acidic agent implicated in chemical ocular injury, does react exothermically with water and can lead to thermal injury of the eye as well.
The last group of chemicals that can lead to chemical ocular injuries are those with neutral pH (6.0 to 8.0). The mechanism of action by which these chemicals affect the structures of the eye varies widely. For example, high dose ethanol can lead to sloughing of the superficial corneal epithelium and damage to the endothelium [13], while sulfur mustard gas has been shown to lead to loss of conjunctival goblet cells and squamous metaplasia [14]. However, on the whole, these agents tend to cause superficial damage primarily due to their relative inability to penetrate into the eye.
Other than pH, other factors to consider are temperature, volume, propensity to form precipitates, velocity of impact, and concentration of the agent, as these also affect the degree of injury and therefore the patient’s long term visual prognosis [10].
Classification
Early grading systems were based on the clinical observation of a linear relationship between the degree of limbal ischemia and prognosis, but Thoft’s classic XYZ hypothesis led to a major paradigm shift suggesting that stem cell survival is the rate limiting mechanism of recovery after every corneal injury [15].
In the absence of a method to directly assess limbal stem cell injury, Wagoner suggested that surrogate measures such as limbal ischemia can be used to estimate of the degree of stem cell loss associated with the injury [10]. Making an accurate estimate if sub-total or total stem cell loss is present is vital as this can predict progression toward sterile corneal ulceration (in the early clinical course) or progressive corneal vascularization and scarring in the late clinical course [10, 16]. These healing patterns seem to be predetermined by the extent of the initial injury and cannot be prevented by optimal medical therapy alone. However, stem cell augmentation with temporary or permanent amniotic membrane transplantation and/or stem cell transplantation can be implemented prior to the onset of these untoward events.
Other less frequent signs and symptoms of MFS include dysesthesias, ptosis, facial weakness, limb weakness, dysphagia, headache, optic neuritis, photophobia, blurred vision, and periocular pain. MFS can present with features that overlap with GBS and other GBS subtypes, on occasion progressing to quadriparesis and respiratory failure requiring mechanical ventilation. In patients with MFS without features of overlap, 1% require assisted ventilation. However, patients with MFS-GBS overlap require mechanical ventilation even more often than patients with GBS alone [5].
There are a myriad of schemes for grading chemical injuries, including the Roper-Hall modification of the Hughes classification scheme (Table 1), Wagoner [10,16], and Dua (Table 2) [10, 17]. They all share the common feature of correlating prognosis with clinical findings that are reflective of the severity of limbal stem cell injury.
|
Grade |
Prognosis |
Cornea |
Conjunctiva/Limbus |
|
I |
Good |
Corneal epithelial damage |
No limbal ischemia |
|
II |
Good |
Corneal haze, iris details visible |
<1/3 limbal ischemia |
|
III |
Guarded |
Total epithelial loss, stromal haze, iris details obscured |
1/3-1/2 limbal ischemia |
|
IV |
Poor |
Cornea opaque, iris and pupil obscured |
>1/2 limbal ischemia |
|
Grade |
Prognosis |
Degrees of limbal involvement (clock hours) |
Degree of conjunctival involvement |
Analogue scale (clock hours / %) |
|
I |
Very good |
0 |
0% |
0/0 |
|
II |
Good |
≤3 |
≤30% |
0.3 to 3/1 to 29.9 |
|
III |
Good |
>3 to 6 |
>30-50% |
3.1 to 6/31 to 50 |
|
IV |
Good/Guarded |
>6 to 9 |
>50-75% |
6.1 to 9/51 to 75 |
|
V |
Guarded/Poor |
>9 to <12 |
>75-<100% |
9.1 to 11.9/75.1 to 99.9 |
|
VI |
Very poor |
Total limbal involvement |
100% |
12/100 |
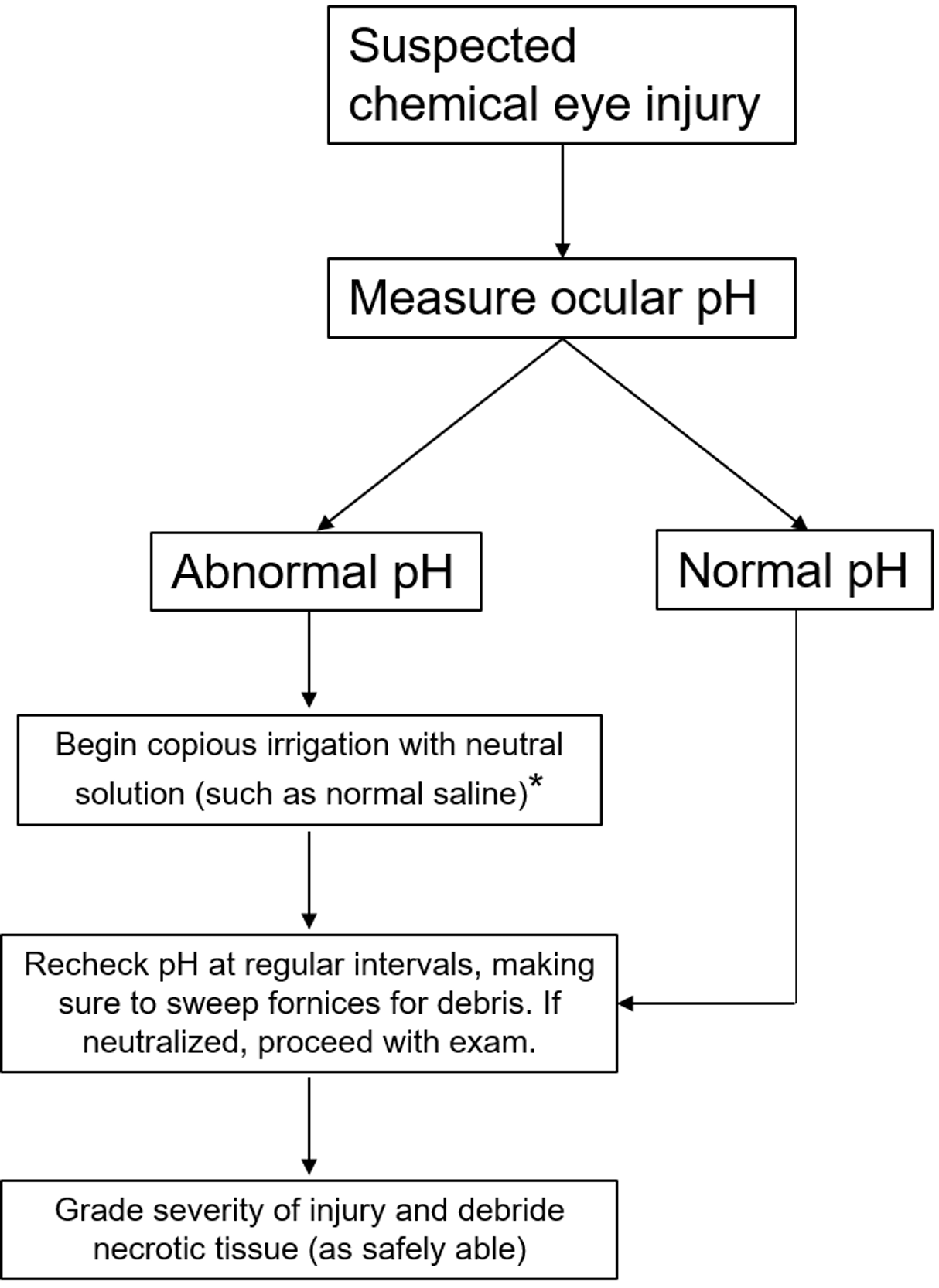
Initial Management (see figure 3)
Before any workup or in-depth examination occurs, it is critical that the patient receives immediate and copious amounts of irrigation to the affected eyes in order to neutralize the pH as quickly as possible. While there may be some advantage to using Cederroth Eye Wash [15] or amphoteric solutions such as Diphoterine [16], the most important factor is reducing time to treatment [10]. Therefore, even before the patient reaches the hospital, irrigation with water, normal saline, or other immediately available non-caustic neutral liquids should be started immediately. The eyes should be irrigated until the ocular surface pH is normalized to between 7.0 to 7.2. When checking ocular pH, it is important to wipe away excess topical anesthetics to get a more accurate measure of the pH. If the patient is unable to tolerate irrigation, a Morgan lens may be used instead. Timely first-line care, often carried out by non-ophthalmologic providers, is essential for preventing long term sequelae [2 ,5 ,10 ,17]. Therefore, it is important to communicate with all providers that immediate and copious irrigation must not be delayed for any reason if a chemical ocular injury is suspected.
After ocular surface pH is neutralized, it should be monitored continuously at 15 to 30 minute intervals to ensure stability. Even if the history is suggestive of unilateral injury, the pH of both eyes must be checked. Examination at that time should focus on identifying any remaining chemical particles and assessing the degree of injury. Eversion of the eyelids and sweeping of the fornices is important for identifying and removing any hidden precipitates. Lime, in particular, forms calcium soaps that can be lodged in the superior fornix and cause severe damage if not removed in a timely fashion [10]. The rest of the ophthalmic examination should include measuring visual acuity and IOP, as well as identifying the degree of conjunctival, corneal, and limbal involvement to help with classifying the extent of injury (Figure 3). Fluorescein can be used to aid in identifying epithelial defects of the cornea and conjunctiva. Examination of the posterior segment may not be possible but should be attempted, with care to use mydriatics without a vasoconstrictive effect as those may exacerbate ischemia damage.
Treatment/Management
For any suspected chemical ocular injury, neutralizing surface pH via aggressive lubrication as soon as possible is universally recommended and key to promoting better patient outcomes (figure 3) [18-20]. The next steps in management focus on closing the epithelium, controlling inflammation, and supporting corneal repair [10 ,17]. Treatment should be based on the initial estimate of the extent of limbal stem cell injury. For injuries with any suspected stem cell injury we recommended aggressive medical therapy. When the evolving course of epithelial recovery suggests the potential for development of sterile corneal ulceration or progressive vascularization, surgical intervention with stem cell supportive or restorative procedures is indicated.
I. Closing the epithelium
When an absence of limbal ischemia (Roper-Hall I) suggests no limbal stem cell injury, liberal use of preservative free lubrication is usually sufficient to promote re-epithelialization. Re-epithelialization in eyes with stem cell injury (Roper-Hall II or greater) may be delayed. Full medical support, and sometimes surgical intervention, is required to facilitate expedited epithelial recovery. Patients should be followed as frequently as needed, which may be on a daily basis in severe cases. To promote epithelial closure, autologous serum drops, bandage contact lens (BCL) placement, and temporary amniotic membrane transplantation (Prokera) can be used to maximize epithelial wound repair by residual limbal stem cell populations. Serum drops include autologous serum eye drops (ASEDs), umbilical cord serum (UCS), and platelet rich plasma (PRP) [21,22]. While UCS and PRP can be more difficult to obtain and standardize, their increased concentration of factors such as epithelial growth factor (EGF), transforming growth factor-beta (TGF-β), insulin-like growth factor-1 (IGF-1), and vitamin A may make them more effective at promoting re-epithelialization [18 ,19].
In cases with profound limbal ischemia and presumptive total or severe sub-total stem loss, Tenonplasty [10,23,24] may be helpful in preventing or treating early-onset sterile ulceration. Tenonplasty can be technically difficult to perform in the acute setting given the tissue friability but may be effective in reestablishing blood supply to ischemic areas of the ocular surface, especially when persistent sterile ulceration and/or necrosis are present. Tenonplasty may be more effective when used in conjunction with ocular surface fluorescein angiography [20]. In cases with sub-total limbal ischemia, delayed re-epithelialization can be addressed with amniotic membrane transplantation and/or limbal stem cell transplantation, although surgical intervention (e.g. Tenonplasty) may be needed as noted above. It is important to stress that these procedures are additive, not replacements for the aggressive medical therapies that are essential to achieve re-epithelialization and counteract ulceration [10,16].
A temporary AMT (Prokera) is a much better choice than a bandage contact lens in the immediate care of serious injuries since it provides comfort and, unlike BCLs, facilitates the rate of epithelial recovery by augmenting the function of residual stem cells [26,27]. The absence of epithelial recovery within 21 days is suggestive of severe stem cell injury and a high risk for sterile corneal ulceration (at worst) or progressive vascularization and scarring (at best). Either way, these impending adverse events are best treated prophylactically with a preemptive limbal stem cell transplantation, preferably with adjunctive AMT [16].
II. Controlling inflammation
Reducing inflammation is important when treating chemical ocular injuries as collagenases and degranulating polymorphonuclear leukocytes (PMN) can slow down epithelial regeneration, and lead to persistent stromal inflammation and sterile ulceration [10]. Current therapeutic strategies for controlling inflammation include prompt debridement of any necrotic conjunctival tissue and use of anti-inflammatory medications. These include topical corticosteroids, topical citrate, topical medroxyprogesterone, and, in some cases, systemic corticosteroids [28-32] In general, strong topical corticosteroids such as difluprednate, prednisolone acetate 1% or dexamethasone 0.1% are recommended for initial treatment as they inhibit collagenase activity and have little risk of sterile ulceration in the first ten days of use [10,16]. However, corticosteroids can interfere with stromal wound repair by impairing keratinocyte migration and collagen synthesis, and therefore increase the risk of sterile ulceration if used for an extended period of time. Instead, medroxyprogesterone may be substituted for corticosteroids at 10 to 14 days [30]. Medroxyprogesterone reduces the risk of sterile corneal ulceration by strongly suppressing collagenase activity while not interfering with collagen synthesis and stromal repair [21]. Topical and systemic citrate also reduces the risk of sterile ulceration by reducing early and late repair phase PMN infiltration [22]. Systemic corticosteroids may also be considered as well if topical agents are not sufficient [23]. The literature does not recommend the use of nonsteroidal anti-inflammatory agents (NSAIDs) as these may delay corneal healing [24] and promote corneal melts in the presence of epithelial defects [25].
III. Promote repair and prevent ulceration
Tetracyclines and ascorbic acid have been found to decrease the risk of ulceration by reducing collagenolysis and promoting repair, respectively. In addition to its antimicrobial actions, tetracyclines reduce collagenase activity, inhibit PMN activity, suppress alpha-1-antitrypsin degradation, and scavenge reactive oxidative species [26]. Oral doxycycline is the most potent tetracycline collagenase inhibitor and is the treatment of choice based on its documented clinical and experimental efficacy [27 ,28]. Ascorbic acid supports stromal repair by serving as a cofactor in the formation of stable triple helix collagen molecules in the stromal matrix as well as through its antioxidant properties [29 ,30]. Anterior segment and stromal ascorbic levels are reduced after stromal and anterior segment penetration of strong alkalis, respectively, but can be restored with topical and systemic supplementation [31]. Oral supplementation alone is probably sufficient in cases where topical use is not well tolerated [32].
IV. Long term sequelae
Chronic complications of chemical ocular injuries include limbal stem cell deficiency, vision-limiting corneal scarring, secondary glaucoma, entropion, and cicatrization of the conjunctiva with symblepharon formation [41].
EPIDEMIOLOGY
|
ETIOLOGY
|
DIAGNOSIS/EMERGENT MANAGEMENT
|
TREATMENT/MANAGEMENT
|
Acknowledgements: The authors are grateful to Dr. Michael D. Wagoner for his assistance in reviewing this manuscript.
References
Yu CY, Diel RJ, Jiang L, Greiner MA. Chemical Eye Injuries: A Case Report and Tutorial. EyeRounds.org. February 28, 2022. Available from https://eyerounds.org/cases/307-chemical-eye-injury.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.