Chief Complaint: Worsening blurry vision in both eyes
History of Present Illness: A 77-year-old woman was referred for worsening vision. Following an uncomplicated cataract surgery, her vision initially improved. However, her vision gradually became blurry and distorted again over the next few months. She denied any acute changes such as pain, discomfort, irritation, redness, glare, dryness, or photophobia. She is pseudophakic in her right eye, has a cataract in the left eye, and has epiretinal membranes in both eyes.
Past Ocular History
Past Medical History
Systemic Medications
Ocular Medications
Allergies
Family History
Social History
Review of Systems
OCULAR EXAMINATION
| Right eye | Left eye | |
|---|---|---|
| Lids/Lashes | Normal | Normal |
| Conunctiva/Sclera | Clear and quiet | Clear and quiet |
| Cornea | 2+ Descemet membrane folds, 4+ guttae, 1+ edema, anterior stromal haze temporal paracentral | Trace Descemet membrane folds, 4+ guttae |
| Anterior Chamber | Deep and quiet | Deep and quiet |
| Iris | Normal architecture | Normal architecture |
| Lens | Posterior chamber intraocular lens, clear | 2+ nuclear sclerosis, 1+ cortical cataract |
| Vitreous | Posterior vitreous detachment (PVD), no anterior vitreous cell (AVC) | Normal, no AVC, no PVD |
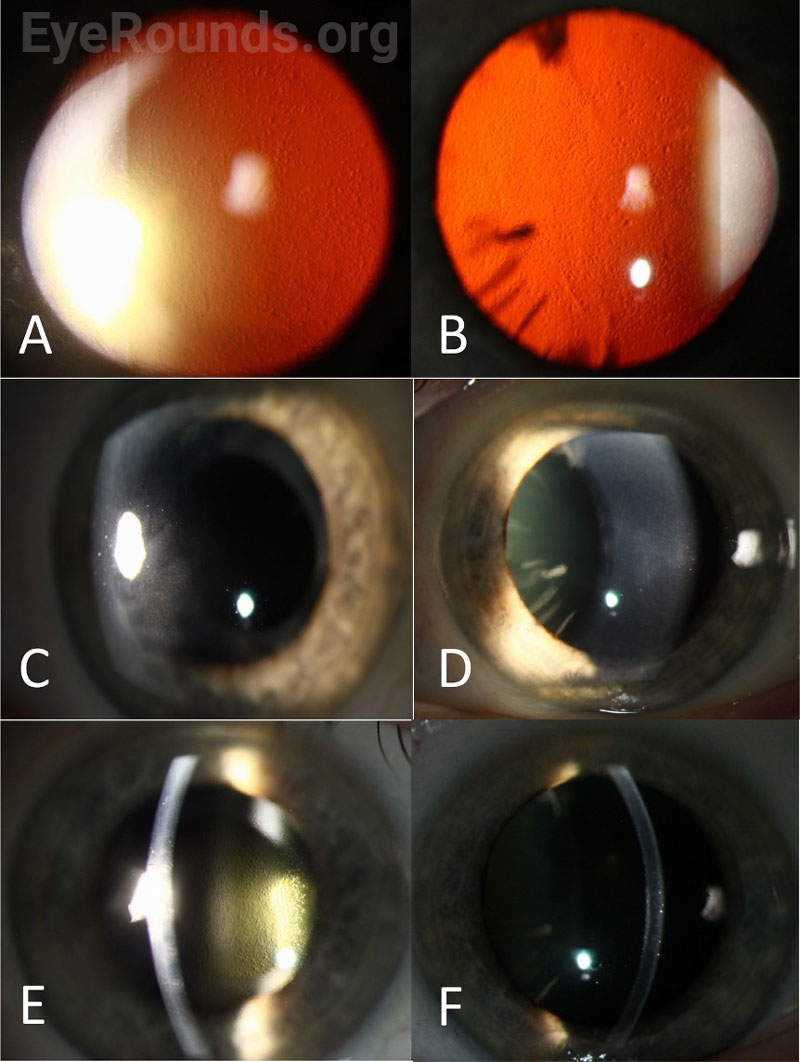
| Right eye | Left eye | |
|---|---|---|
| Disc | Normal, healthy rims | Normal, healthy rims |
| Cup-to-disc ratio | 0.2 | 0.2 |
| Macula | Mild epiretinal membrane | Trace epiretinal membrane |
| Vessels | Normal | Normal |
| Periphery | Normal | Normal |
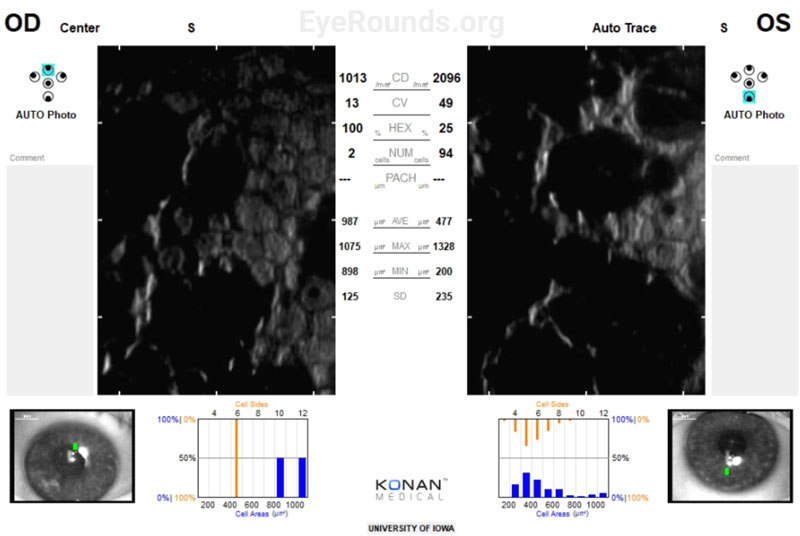
Specular Microscopy:
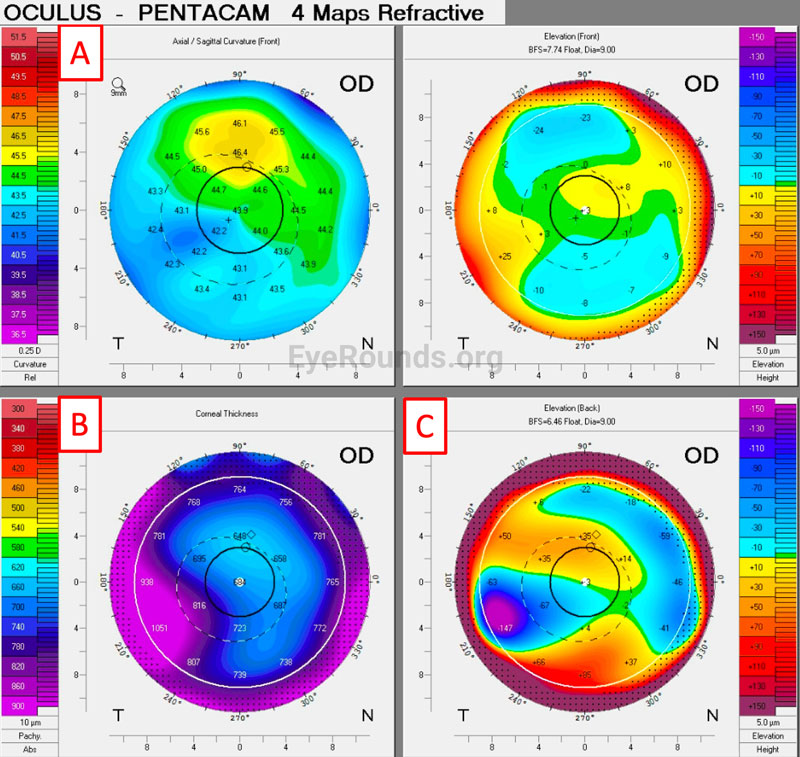
Corneal Tomography:
Differential Diagnosis
DIAGNOSIS: Fuchs’ Endothelial Corneal Dystrophy (FECD)
CLINICAL COURSE
The patient was diagnosed with decompensated Fuchs’ endothelial corneal dystrophy with significant corneal edema in both eyes, worse in the right eye than the left. She had initially preferred medical management and had been on Muro 128 for two years with regular follow-up. Despite this, her vision had continued to worsen, and the disease had progressed to the point where the patient was ready to consider surgical options.
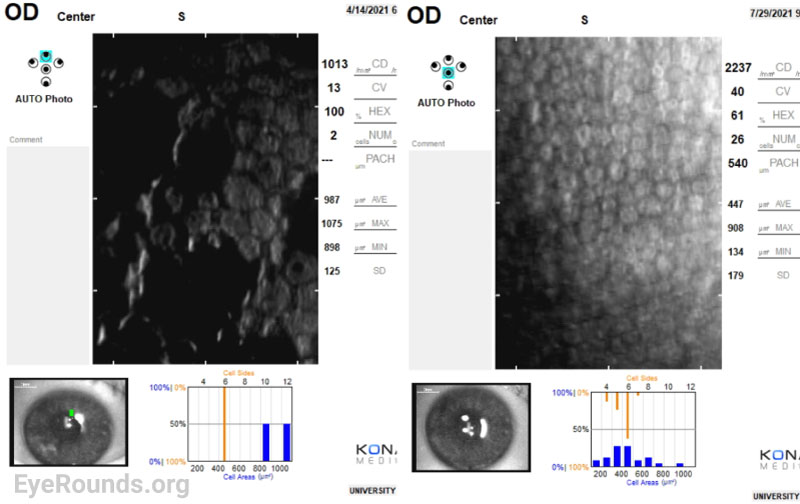
The decision was made to proceed with a partial thickness corneal transplant, Descemet Membrane Endothelial Keratoplasty (DMEK), in her right eye. Three months following the procedure, her visual acuity in the right eye improved from 20/30-1 to 20/25+1 (with pinhole correction to 20/20-1). Her central corneal thickness also decreased from 670 µm to 557 µm, indicating improved corneal endothelial cell function. However, her endothelial function in the left eye continued to decompensate with visual acuity worsening to 20/200 (pinhole 20/70+2). The decision was made to first undergo cataract surgery followed by DMEK a month later. A week after her cataract extraction, her visual acuity in the left eye was 20/60-1. At her one-month post-operative appointment following DMEK, her best corrected visual acuity had improved to 20/30-2 (pinhole correction to 20/20-2). Her corneal thickness map and specular microscopy normalized, indicating a successful DMEK with improved endothelial function.
Specular Microscopy:
Corneal Tomography:
DISCUSSION
Etiology/Epidemiology
Fuchs’ endothelial corneal dystrophy (FECD) is the most common primary corneal endothelial dystrophy, affecting 4-7% of individuals over 40 years old [1,2]. FECD can be divided into two subtypes: early-onset and late-onset (Table 1). Early-onset FECD usually presents in the 2nd or 3rd decade of life and is highly associated with an autosomal dominant COL8A2 mutation [3,4]. Late-onset FECD has a greater predilection for females than males (3-4:1 ratio) and is associated with trinucleotide repeats in the intronic region of TCF4 or with mutations in SLC4A11 or ZEB1 [5]. TCF4 trinucleotide repeats have been reported occur in up to 70% of FECD cases and are believed to cause RNA toxicity through alteration of splicing machinery [5]. In addition to genetic predisposition, exogenous factors including smoking, diabetes, UV light, and hormones, specifically estrogen, have all been shown to increase the risk and progression of FECD [6].
| Early-Onset (Rare) | Late-Onset (Common) | |
|---|---|---|
| Female: Male | 1 : 1 | 3-4 : 1 |
| Onset | 2nd or 3rd decade | 5th or 6th decade |
| Genetics |
|
|
| Exogenous Risk Factors | Smoking, diabetes, UV light, hormones [6]] | |
Table 1: Characteristics of Early-Onset vs. Late-Onset FECD
Pathophysiology
The corneal endothelium provides nutritional support and appropriate hydration to the cornea through active regulation of various transport channels. In particular, the endothelium contains Na+/K+ ATPase pumps that transport water out of the corneal stroma into the aqueous humor in order to maintain transparency and prevent corneal edema [7]. In FECD, this endothelial layer becomes dysfunctional due to accumulations of extracellular matrix material that collect in guttae within Descemet membrane and extend posteriorly, disrupting the endothelium. Pathogenic genetic mutations and exogenous stressors promote FECD through improper protein function and misfolding, endoplasmic reticulum and oxidative stress, and mitochondrial dysfunction that lead to endothelial cell death and senescence and improper functioning of the transport channels [8].
Signs & Symptoms
Most patients with FECD are completely asymptomatic and are diagnosed during routine clinical examination through visualization of guttae on slit lamp examination. As the disease progresses, patients will complain about reduced vision and halos, worse in the morning upon waking. Diurnal fluctuation in visual acuity results from buildup of corneal edema while the eyelids are closed with symptomatic eyes have substantially thicker corneas than asymptomatic ones (9). In more advanced disease, patients will have decreased vision, photophobia, epiphora, and pain due to rupture of epithelial bullae leading to epithelial defects and ulcers.
The modified Krachmer Grading Scale (Table 2) is routinely used in clinical practice to grade the severity of FECD and is largely based on the number and confluence of guttae visible on retroillumination [10]. Guttae are composed of spindle-shaped bundles of wide-spaced collagen, which give the cornea a beaten metal appearance with dark round areas on Descemet membrane that disrupt the endothelium [11]. Guttae first accumulate in the center of the cornea and spread peripherally as the disease progresses, increasing in number an average of 29% over a 30 month period [12].
| Grade | Retroillumination or slit-lamp biomicroscopy appearance | Symptoms | Treatment | |
|---|---|---|---|---|
| Mild | 1 | ≤ 12 central nonconfluent guttae | None | Observation |
| 2 | < 12 central nonconfluent guttae | Mild reduction of vision |
|
|
| Moderate | 3 | 1-2 mm central confluent guttae | Reduced vision and halos in the morning | |
| 4 | 2-5 mm central confluent guttae | |||
| Severe | 5 | > 5 mm central confluent guttae without edema | Reduction in vision, photophobia, epiphora, pain if rupture of bullae |
|
| 6 | >5 mm central confluent guttae with edema |
Table 2: The modified Krachmer Grading Scale for the clinical assessment of disease severity in FECD with associated signs, symptoms, and treatments for each grade [8,13]
Additional testing
Disease progression can be further assessed using specular or confocal microscopy to visualize endothelial cell dropout, accumulation of guttae, and polymegathism/pleomorphism of the remaining corneal endothelial cells. The average endothelial cell density for most adults over 40 is between 2000-3000 cells/mm [2].
Scheimpflug tomography pachymetry and posterior elevation map patterns can be used to assess disease and predict progression. This can be especially helpful when there is not clinically appreciable edema present [13]. Patel et al. determined that findings predictive of progression in FECD include loss of isopach concentricity, displacement of the thinnest point of the cornea, and focal posterior surface depression. In isolation, central corneal thickness is not a helpful prognostic tool [14].
Treatment & Management
Initial management in asymptomatic individuals is often observation. In those with mild to moderate visual disability, medical management can include topical NaCl 5% drops up to 4 times per day and ointment at night to increase external osmolality, thereby dehydrating the corneal epithelium. In those with ruptured bullae, the epithelial defect should be treated as a corneal abrasion.
With progression of disease and further visual disability, surgical treatment via corneal transplant should be considered. While penetrating keratoplasty (PK) surgery was historically the mainstay of treatment, endothelial keratoplasty with Descemet membrane endothelial keratoplasty (DMEK) or Descemet stripping automated endothelial keratoplasty (DSAEK) have surpassed this over the past two decades. Patients undergoing endothelial keratoplasty tend to have greater improvement of visual acuity, less postoperative astigmatism, faster visual recovery, and greater refractive stability in comparison to patients undergoing PK (15 ,16). DSAEK and DMEK also have superior graft survival with 96.2% and 98.7%, respectively, compared to a 73.5% survival rate of PK grafts [17] and do not require as potent of immunosuppressive treatment. A more detailed description of DSAEK and DMEK can be found here.
Treatment alternatives to corneal transplantation are actively being investigated. Descemet stripping without endothelial keratoplasty, otherwise known as “Descemet Stripping Only” (DSO) has shown promising outcomes in select patient groups. In DSO, the central Descemet membrane and corneal endothelium are surgically removed. Postoperatively, peripheral endothelial cells migrate to the region that was originally stripped, creating a new barrier which helps to treat and prevent edema [18]. Disadvantages of DSO include longer visual recovery compared to EK, need for healthy peripheral endothelial cells, and lack of long-term reported outcomes. Other studies have shown encouraging data demonstrating accelerated endothelial cell migration following DSO with the use of topical rho-associated coiled-coil-containing protein kinase (ROCK) inhibitors, like ripasudil [19 ,20].
Injection of donor cultured endothelial and pluripotent/multipotent stem cells is also actively being studied via human clinical trials. Gene therapy is another potential avenue for treating FECD, and it is actively being investigated via the CRISPR/Cas9 system. Pharmacologic intervention for FECD is also on the rise to slow FECD progression and reduce the need for surgery, with several molecules under investigation in preclinical and early phase clinical trials [21 ,22]. While various treatment modalities for FECD currently exist, further investigation may provide better outcomes, safety protocols for patients, and better overall access to care.
ETIOLOGY/EPIDEMIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT/MANAGEMENT
|
Field MG, Garza Reyes G, Dotson AD, Witsberger EM, Sales CS, Greiner MA. Fuchs’ Endothelial Corneal Dystrophy. EyeRounds.org. Posted August 7, 2023; Available from https://EyeRounds.org/cases/343-fuchs-endothelial-dystrophy.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.