
The University of Iowa
Department of Ophthalmology and Visual Sciences
June 14, 2017
Part of the complete ophthalmic examination includes inspection of the eyelids and lashes. Anatomically, the eyelids are bordered superiorly by the eyebrow and inferiorly by the cheek. The majority of this area is covered superficially by a keratinizing stratified squamous epithelium. Because of this, the eyelid is prone to many of the same dermatologic lesions found elsewhere on the skin covered areas of the body.
The eyelids contain numerous specialized adnexal structures that differ depending on the location in the eyelids. The dermis lies deep to the epidermis and contains cilia, the sebaceous glands of Zeis, the apocrine sweat glands of Moll, eccrine sweat glands, and pilosebaceous units. Deep to the orbicularis near the eyelid margin lays a dense plaque of fibrous connective tissue known as the tarsal plate, which contains sebaceous meibomian glands. Finally the conjunctiva contains the accessory lacrimal glands of Wolfring and Krause as well as goblet cells. This tutorial details the common, benign lesions found on the eyelid. Malignant lesions of the eyelid (e.g., basal cell carcinoma, squamous cell carcinoma, sebaceous adenocarcinoma, malignant melanoma, etc.) are covered in a separate tutorial. This article is by no means an extensive discussion of every benign eyelid lesion; rather it serves as an overview/tutorial to guide diagnosis and treatment.
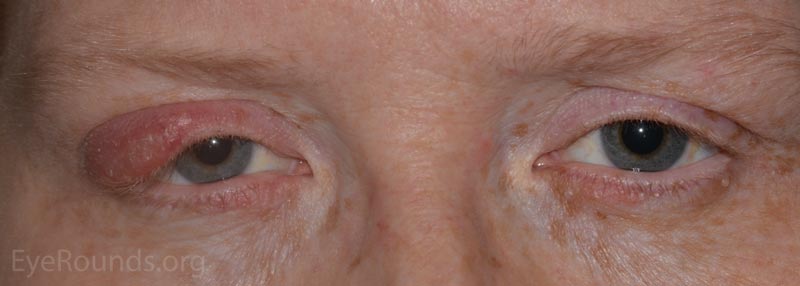
Patients will present with a hard, painless nodule in the eyelid that slowly enlarges over the course of weeks to months. It may be the result of a hordeolum (see note below) or develop de novo. This process is commonly associated with rosacea and blepharitis.
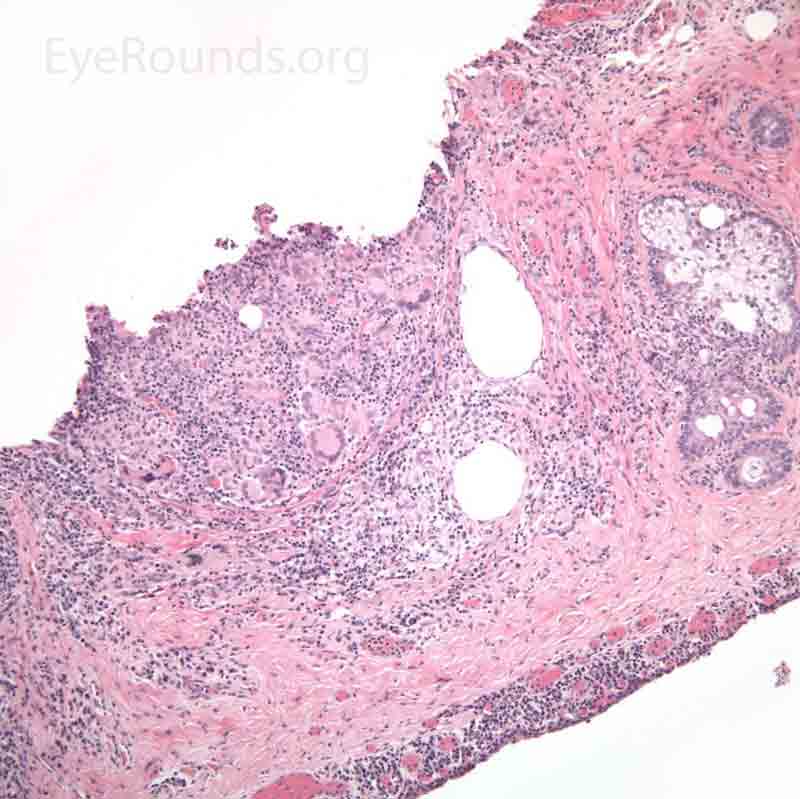
Pathology specimens classically show zonal lipogranulomatous inflammation centered on clear spaces previously filled with lipid ("lipid dropout" – an artifact of processing). There is a mixed inflammatory infiltrate that consists of neutrophils, plasma cells, lymphocytes, epithelioid histiocytes and multinucleate giant cells.
In contrast to a chalazion, a hordeolum (stye) is an acute, purulent inflammatory process of any gland (meibomian, Ziess, Moll, or eccrine) in the eyelid that presents as a discrete, warm, erythematous, painful pustule over the course of a few days. The pathology is typified by a small, purulent abscess consisting of neutrophils and necrotic cellular debris centered on a hair follicle and its adjacent gland.
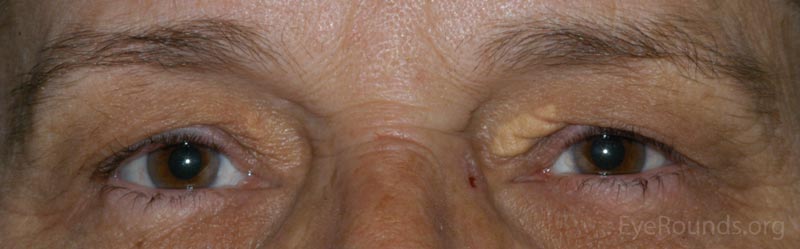
A Xanthelasma is a tumor consisting of intracellular accumulation of lipid. This lesion is typified by a collection of lipid-laden macrophages within the dermis.
The patient will present with multiple soft, yellowish plaques commonly found near the medial canthi of the upper and lower lids. These lesions are more common with increasing age and may be associated with disorders of lipid metabolism.
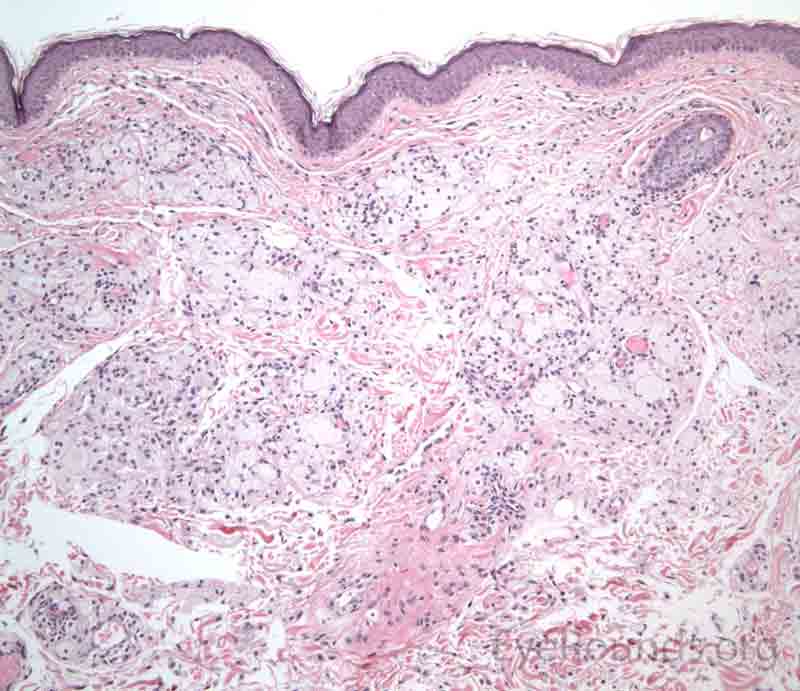
The dermis will show a collection of histiocytes with foamy, lipid-laden cytoplasm that tend to cluster around blood vessels.
There is a high recurrence rate after treatment for xanthelasma.
An epidermal inclusion cyst (EIC) is a dermal implantation cyst of epidermis. It can be congenital or acquired. The acquired form is usually in a site of prior trauma, which causes occlusion of the orifice of the hair follicle.
This often presents as a slow-growing, elevated, round, smooth, white lesion. These lesions do not trans-illuminate and can have a central pore that designates the remaining pilar duct. These lesions can become secondarily infected or rupture and incite an inflammatory reaction.
A pathologic specimen of this process will show a cystic structure within the dermis that is lined by stratified squamous keratinizing epithelium with desquamated keratin in the cyst lumen. There are no dermal appendages in the cyst wall (this is the differentiating feature from a dermoid cyst).
Milia are multiple, small epidermal inclusion cysts that are histologically identical to EIC and vary only in size. Cutaneous (not orbital) dermoid cysts are similar to EIC, but contain skin adnexal structures (hair, sweat/sebaceous glands) in the cyst wall. The cyst lumen also contains hair shafts and glandular secretions in addition to keratin.
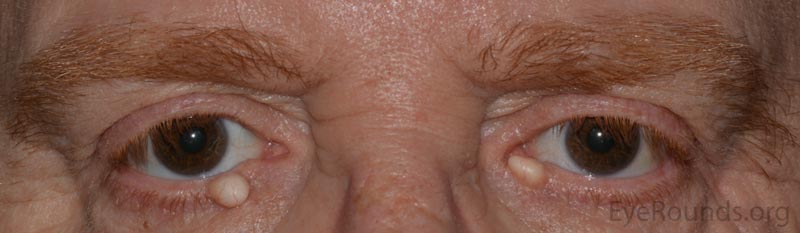
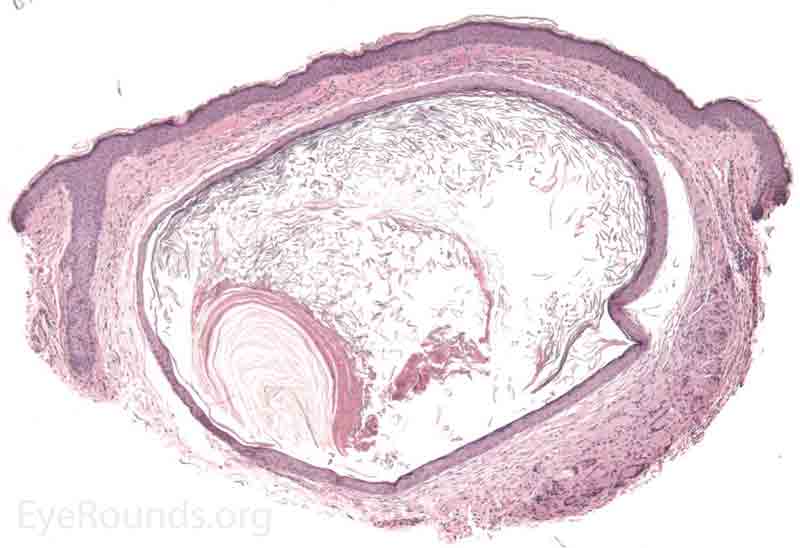
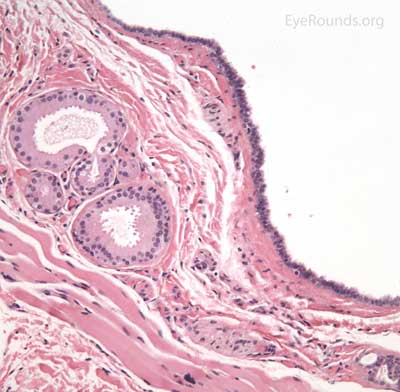
An apocrine hidrocystoma is a cyst that results from ductal occlusion of an apocrine sweat gland of Moll. It is considered a variant of an adenoma of the secretory cells of Moll rather than a retention cyst.
The patient will present with a solitary, round, smooth, cystic lesion located along the lid margin and commonly found near the canthi. These lesions are translucent and will trans-illuminate, but can occasionally take on a bluish tint.
Pathology shows an irregular cystic structure within the dermis. The cyst is lined by a double layer of cuboidal epithelium with the inner-most (luminal) layer demonstrating apocrine differentiation (apical decapitation secretion)..
Eccrine hidrocystoma is a ductal retention cyst resulting from occlusion of a duct of an eccrine sweat gland. These lesions are clinically and sometimes histologically indistinguishable from an apocrine hidrocystoma. These lesions are different in that they enlarge in conditions that stimulate perspiration (heat or humidity) and vary histologically as the cyst lumen is lined with a double layer of cuboidal epithelium without apocrine differentiation.
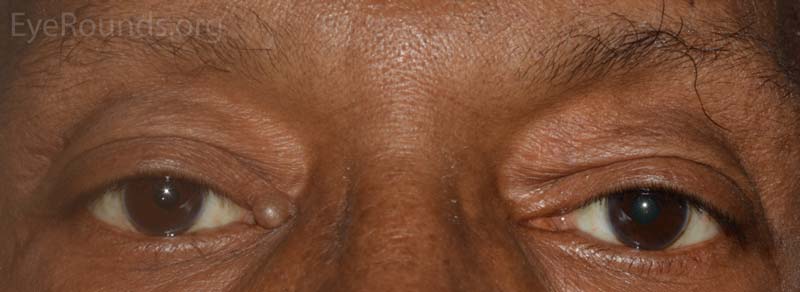
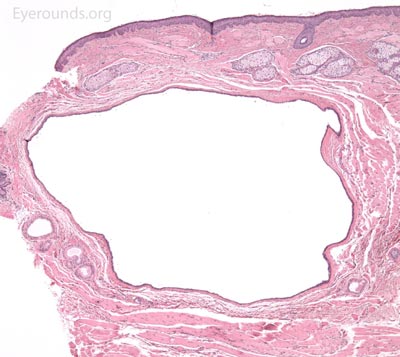
A syringoma is a benign, adenomatous tumor of the eccrine sweat gland that likely arises from malformed eccrine ducts.
The most common presentation is multiple, soft, small (1-2 mm), mildly hypopigmented papules arising on or near the lid margin or in the dermis. Syringoma are more common on the lower lid and occur more often in young female patients.
A pathologic sample of this process will show epithelial strands of small basophilic cells extending into the dermis that represents proliferation of eccrine sweat gland structures. These are classically described as "comma-shaped" or "tadpole" in appearance. Additionally, there will be multiple small, round, cystic ductules of proliferating eccrine glands that are lined by a double layer of flattened epithelial cells with a colloidal secretory material in the central lumen.
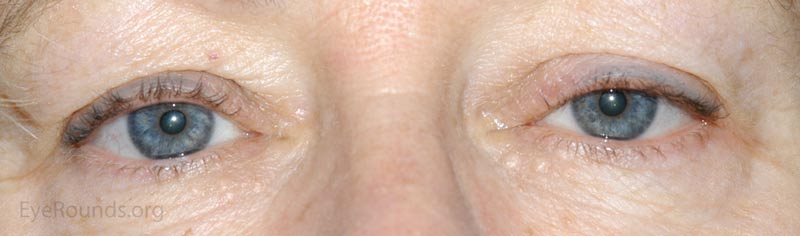
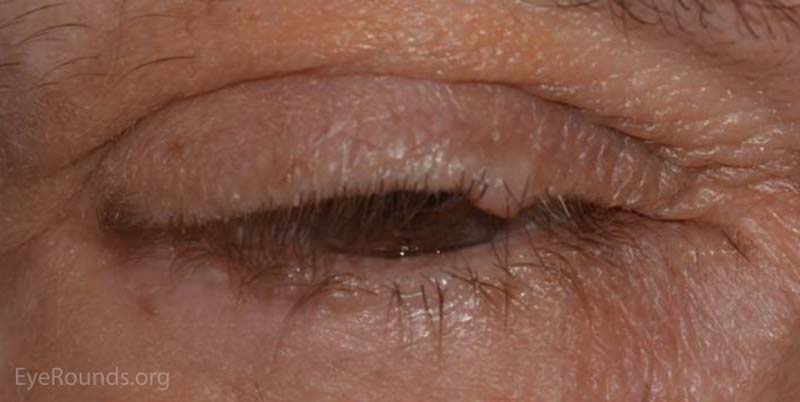
A nevus is a congenital, hamartomatous (benign neoplasm in the tissue of origin) tumor of incompletely differentiated melanocytes (nevus cells).
The presentation of a nevus is highly variable. Though not clinically apparent, nevi are present at birth and typically evolve and manifest variably throughout a person's life. Initial clinical presentation occurs during childhood as a flat, pigmented macule. At this time the nevus is typically a junctional nevus – the nevus cells are located in the basal epithelial layer at the epidermal, dermal junction. Pigmentation often increases during puberty and then beyond the second decade, it becomes an elevated, pigmented papule. Over time the nevus transforms into a compound nevus – the nevus cells have extended from the junctional zone down into the dermis that gives it elevation. As the patient ages, the nevus loses its epidermal pigmentation and remains as an elevated, minimally pigmented or amelanotic lesion. At this point, the nevus is known as an intradermal nevus – there is involution of the epidermal component and all of the nevus cells are within the dermis.
Nevi are frequently found on the periocular skin, eyelids and eyelid margins. Nevi found on the lid margin can mold to the underlying ocular surface if they contact the globe and can have lashes protruding from them.
Just as clinical presentation varies, pathologic features vary depending on the evolutionary stage of the nevus. Typical nevus cells are bland, benign appearing, but atypical melanocytes are round, basaloid and tend to cluster together in nests or chords. These cells contain "pseudo-inclusion cysts" which are abnormal infoldings of the cell nucleus that appear as a clearing within the cell nucleus. Nevus cells tend to show polarity within a lesion, that is the nuclei tend to become more "mature" (smaller, thinner, and darker) as they progress deeper into the dermis. In the superficial aspect of the nevus, type A nevus cells have an epithelioid appearance. The nevus cells become smaller and darker as they move deeper (type B cells). In the deepest aspect of the nevus, type C nevus cells have a flatter, thinner nucleus and take on a spindle or Schwann cell-like appearance. Nevi contain highly variable amounts of pigmentation. As previously described, the location of the nevus cells within the lesion is what classifies the type of nevus
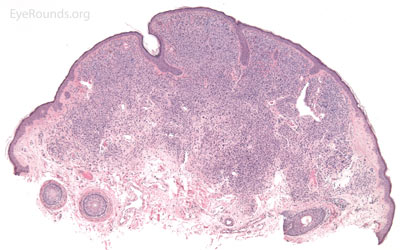
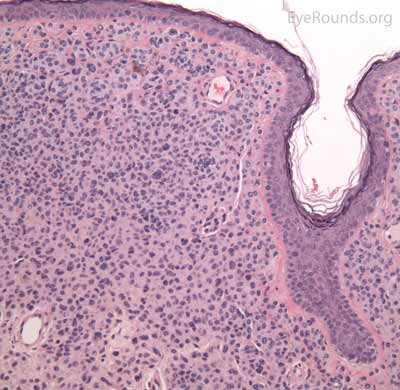
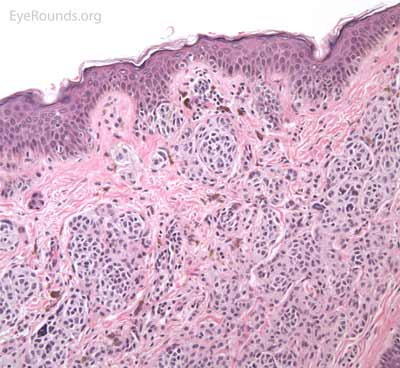
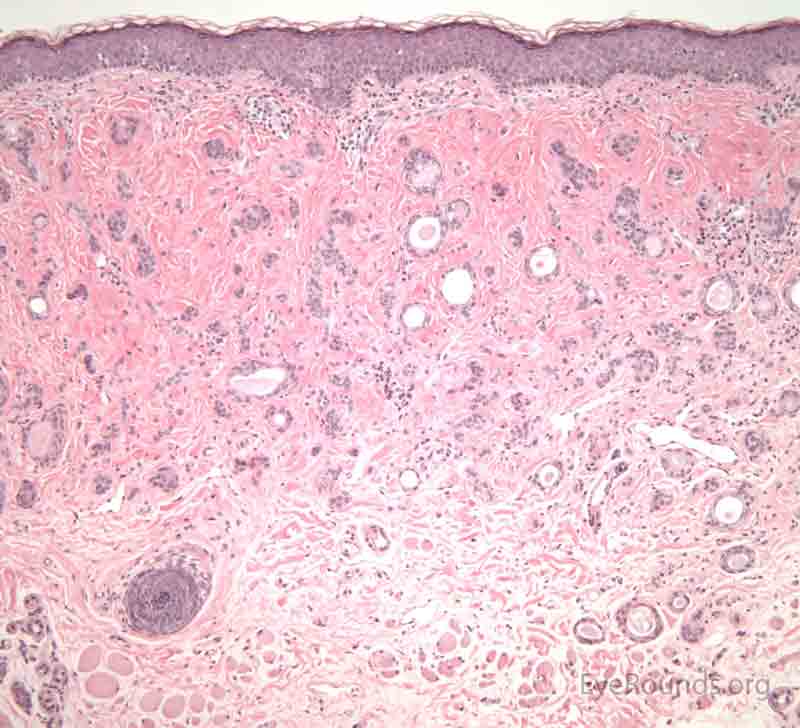
A seborrheic keratosis is an acquired, benign papilloma that results from intraepidermal proliferation of benign basal cells.
The presentation is variable, but lesions are typically sharply defined, brownish and have a rough, warty surface. They are classically described as "greasy" and "stuck-on". The lesions have a variable degree of pigmentation and hyperkeratosis. The morphology may be sessile, pedunculated, lobulated, papillary or verrucoid. It is common for these lesions to increase in size and number with age.
Pathologic specimens will show acanthosis, hyperkeratosis, and papillomatosis. Low magnification will accentuate the "stuck on" appearance of this papillomatous growth with upward acanthosis (Figure 14A). Higher magnification shows a proliferation of cells within the epidermis that closely resemble normal basal cells. The epidermis may proliferate down in to the dermis in a reticulated pattern with narrow interconnecting cords or tracts. There may be pseudohorn cysts, which are crevices or infoldings of epidermis cut in cross-section that appear to be cystic accumulations of keratinous material (Figure 14B). Pigmentation of these lesions is variable.
A sudden onset of multiple seborrheic keratoses is known as Leser-Trélat sign and is associated with systemic malignancy, classically gastrointestinal adenocarcinoma. There is a lesion very similar to seborrheic keratosis known as irritated seborrheic keratosis or inverted follicular keratosis. These lesions typically present as pink to flesh colored small papules that appear with rapid growth. Pathologically they are very similar as well, except that the normal basaloid cells of the lesion surround whorls of non-keratinizing squamous epithelium known as "squamous eddies" within the epidermis.
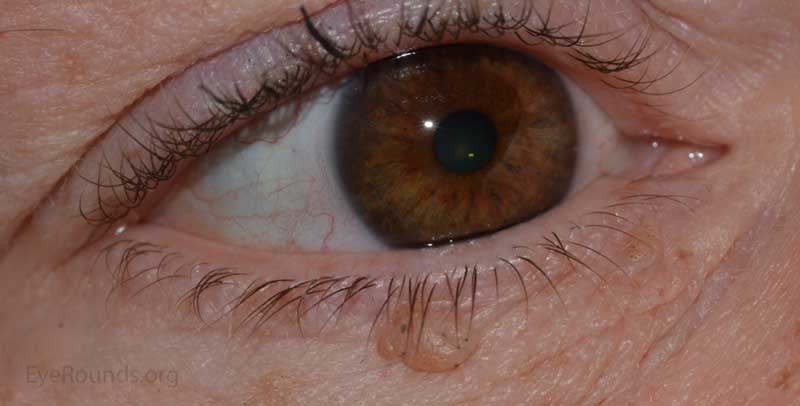
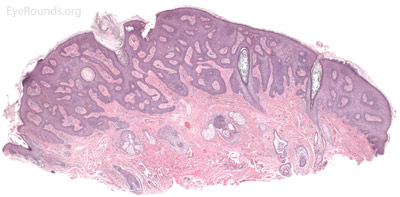
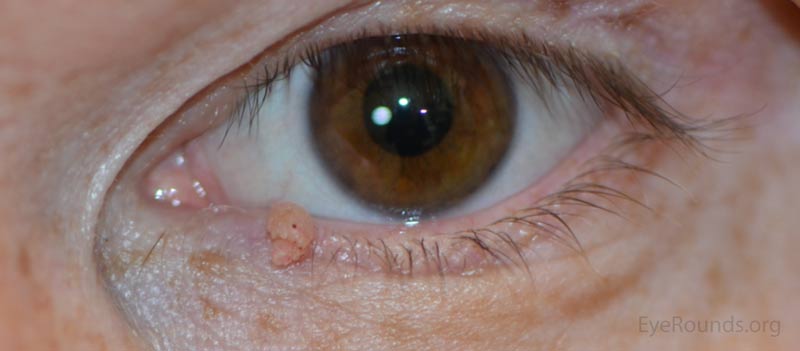
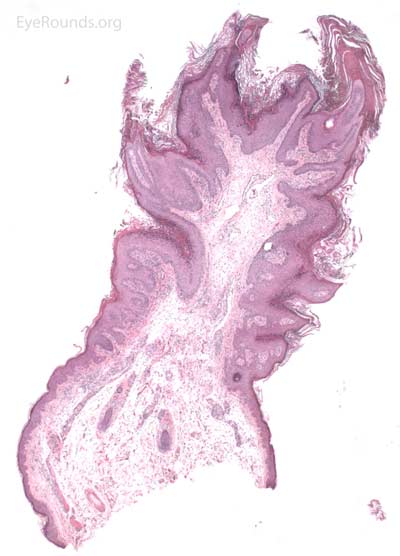
Verruca vulgaris, more commonly known as a wart, is a papillomatous growth that is caused by an epidermal infection with human papilloma virus (usually HPV 6 or 11).
These lesions typically occur near the eyelid margin, but can occur anywhere on the periocular skin. They typically appear as a small, non-pigmented papule with a digitated surface or as an elongated, filiform lesion with papillomatous growth.
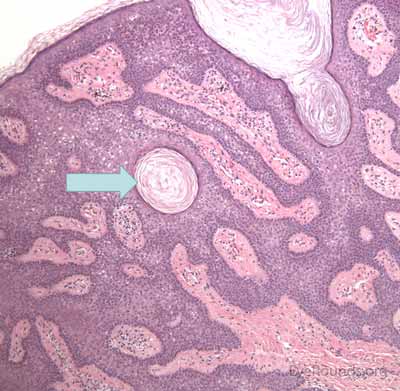
Verruca lesions are typified by massive papillomatosis with acanthosis. There is usually a large degree of hyperkeratosis and these lesions will demonstrate parakeratosis. The parakeratosis in verruca lesions is classically apical (Figure 16A). On higher magnification, infected cells will demonstrate koilocytosis – cytoplasmic clearing with nuclear contraction (Figure 16B). These vacuolated keratocytes will have condensation and clumping of dark-staining keratohyaline granules in the periphery of the cell and occasionally show intranuclear eosinophilic viral inclusion bodies. Verruca lesions will typically have a mixed inflammatory infiltrate in the underlying superficial dermis.
Verruca lesions are known for recurrences.
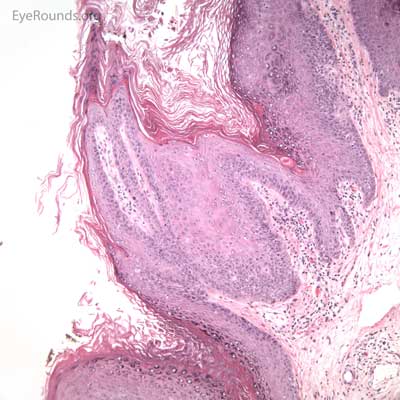
Molluscum contagiosum is an epidermal viral infection caused by the DNA poxvirus Molluscum Contagiosum Virus (MCV)..
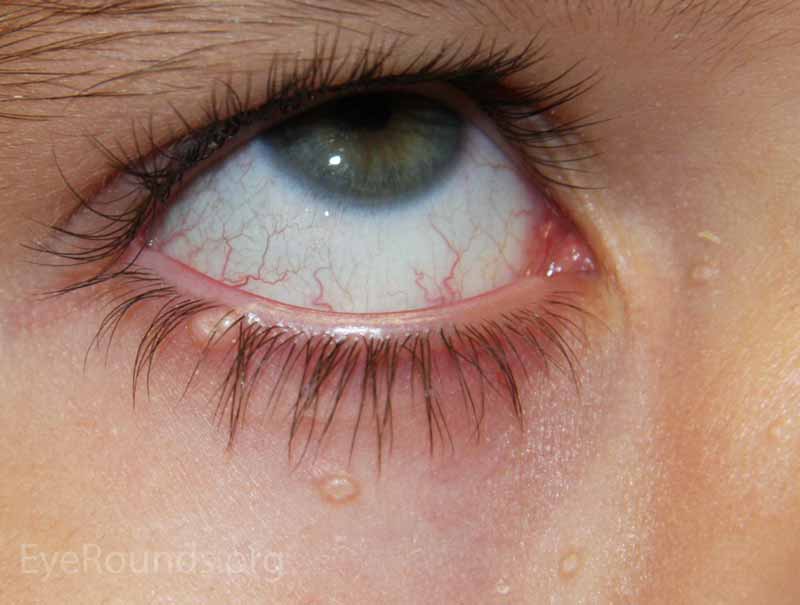
This process will typically present with multiple, small (1-3 mm), discrete, dome-shaped or nodular, waxy papules with characteristic umbilicated centers. It is more common in pediatric populations or those that are immune compromised (i.e., HIV, AIDS, Wiskott-Aldrich syndrome). Patients may also present with a chronic follicular conjunctivitis that is caused by molluscum bodies being shed into the tear film from the eyelid lesions.
This process has a very distinct pathologic appearance of a nodular proliferation of epithelium producing a central focus of necrotic cells extruding to the surface. Infected cells contain large, homogeneous, intracytoplasmic inclusion bodies called molluscum bodies that represent replicating pox virus in the cytoplasm and tend to displace the cell nucleus peripherally (Figure 18B). Infected cells tend to be smaller and more eosinophilic in the deeper layers of the lesion and become larger and more basophilic as they extend toward the surface.
Treatment of the skin lesions will resolve any associated irritative follicular conjunctivitis.
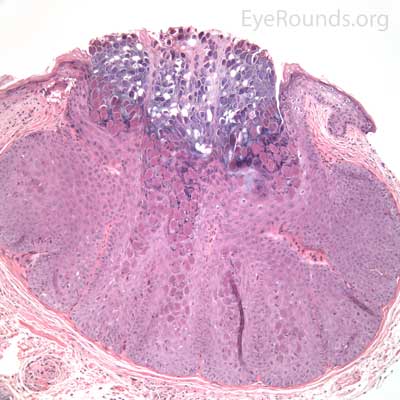
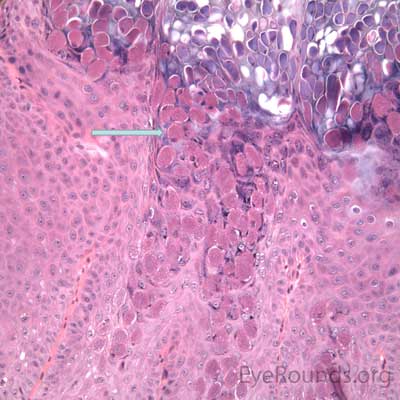
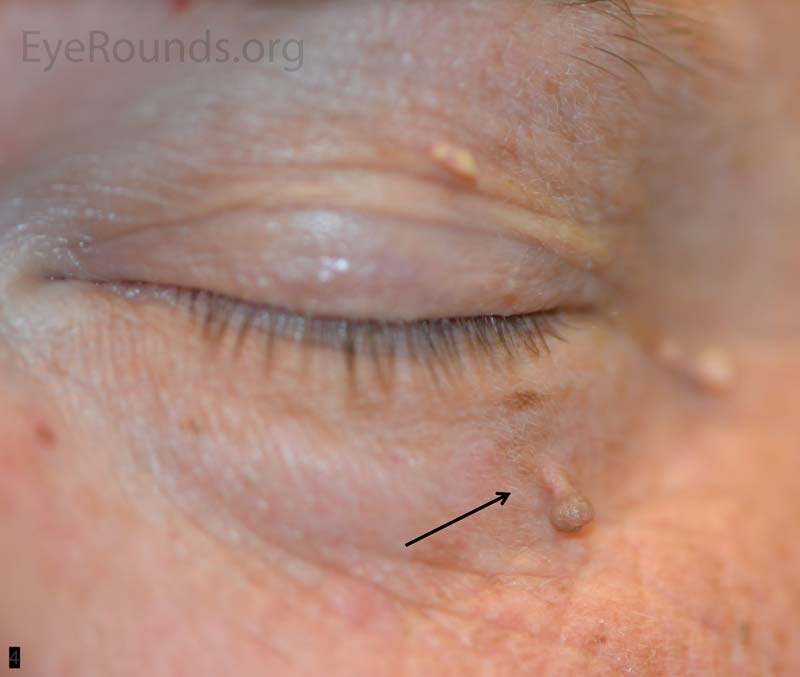
An acrochordon is a benign, acquired papilloma. It is known by many other names including skin tag, fibroepithelial polyp or squamous papilloma.
These lesions can present as single or multiple and can range in size typically from 1 mm to 1 cm. They are classically polypoid, soft and attached by a stalk. The lesions can be non- or slightly pigmented, but if twisted about its stalk, the lesion may infarct and change color from tan to black. They are more common in the middle-aged and elderly patient and have a higher occurrence in areas of skin friction.
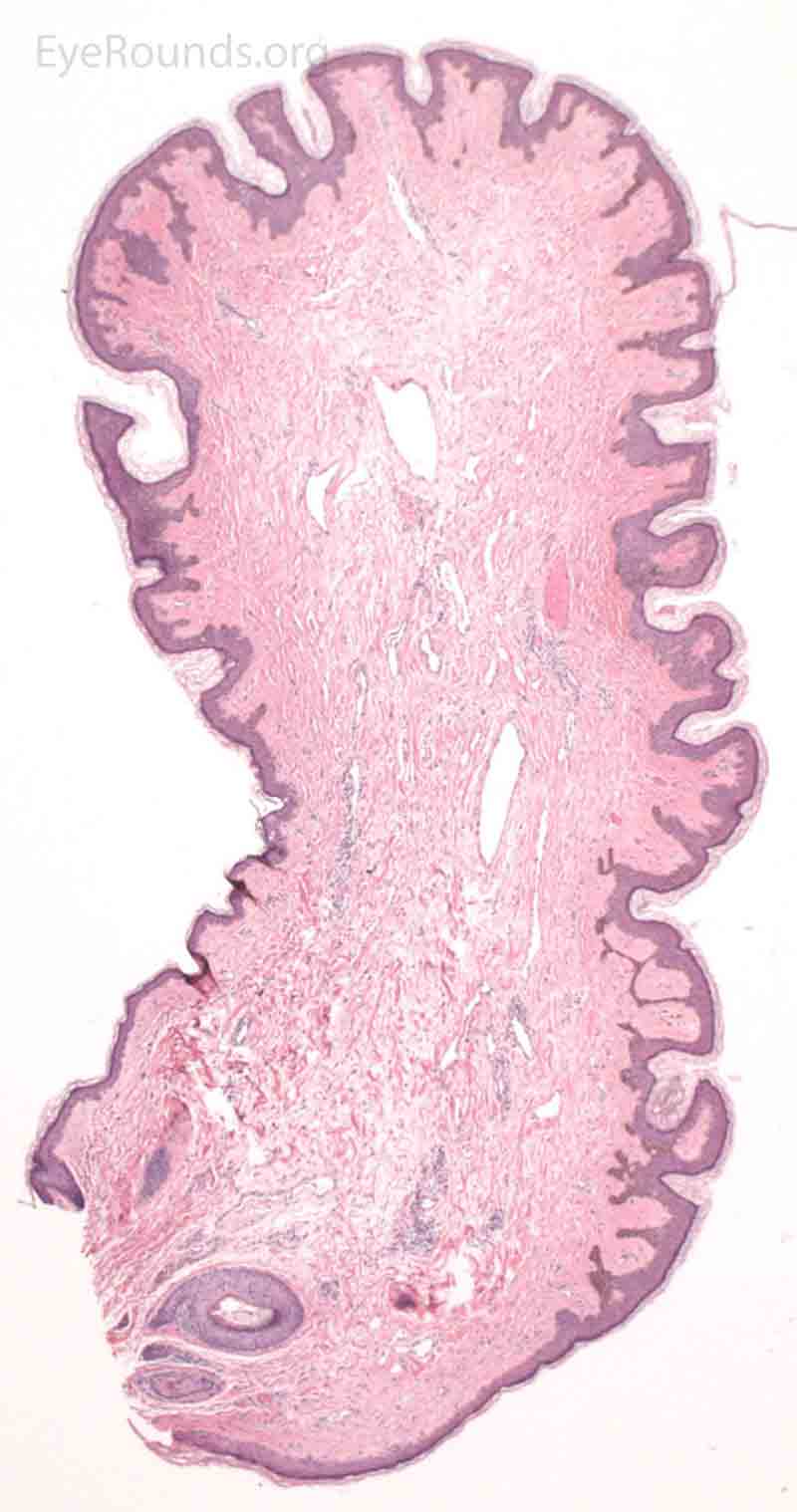
Pathologic specimens show a polypoid lesion with a fibrovascular stalk that contains loose, collagenous stroma surrounded by a mostly unremarkable epithelium. There are varying degrees of acanthosis and hyperkeratosis.
Lenci LT, Kirkpatrick CA, Clark TJ, Maltry AC, Syed NA, Allen RC, Shriver EM. Benign Lesions of the External Periocular Tissues: A Tutorial. EyeRounds.org. posted May 10, 2017; Available from: https://eyerounds.org/tutorials/benign-lid-lesions/index.htm