
The University of Iowa
Department of Ophthalmology and Visual Sciences
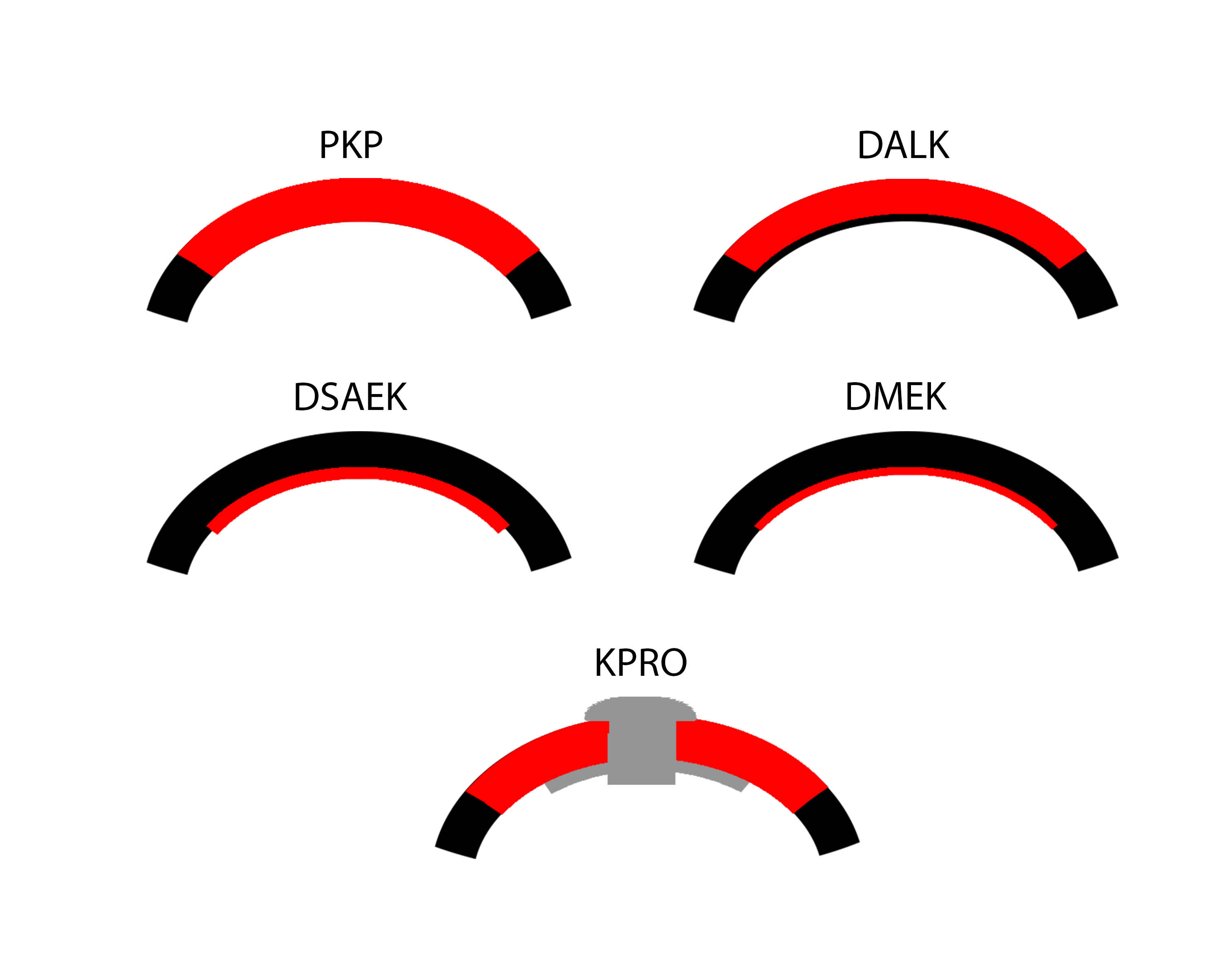
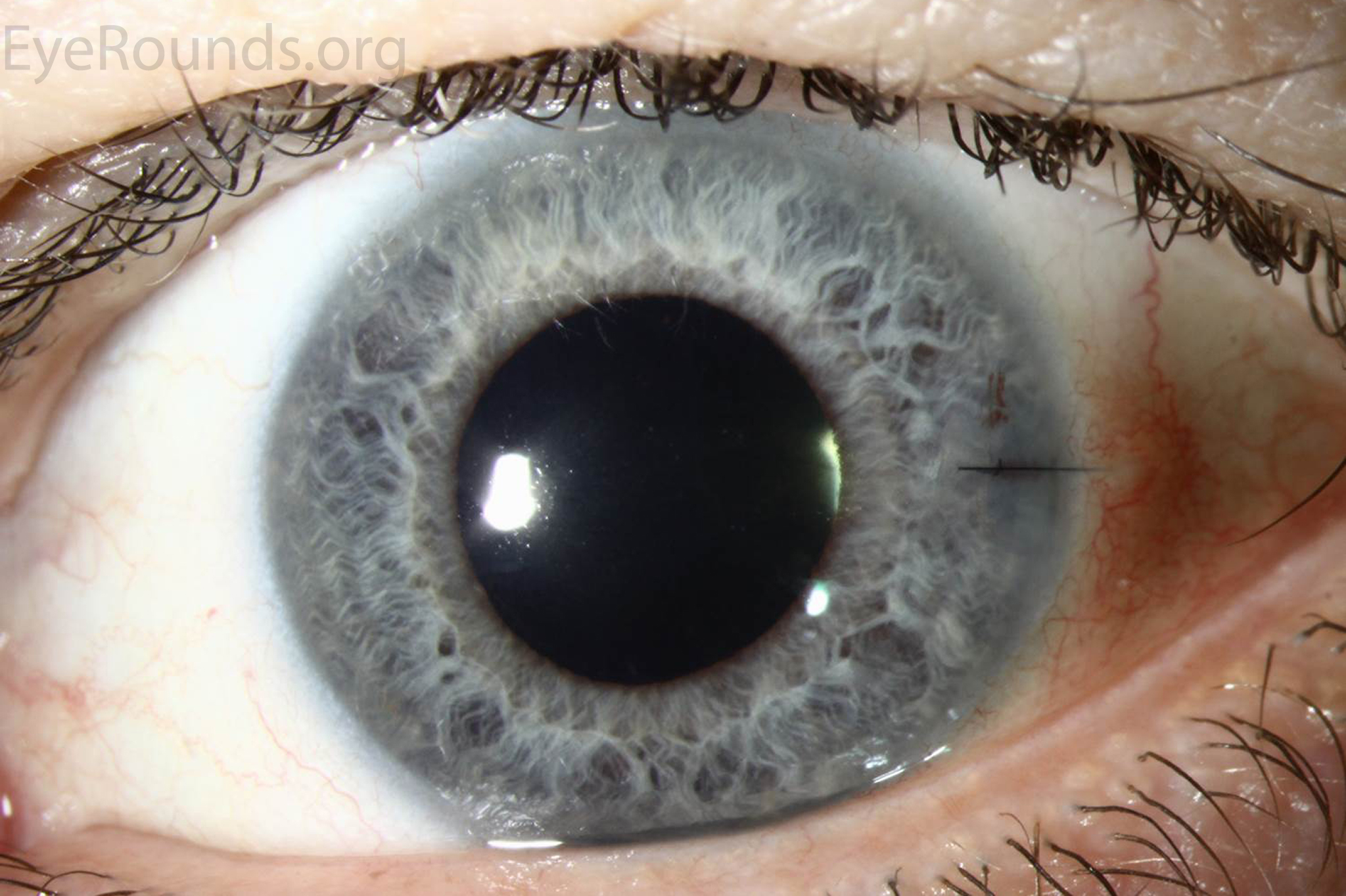
While full-thickness corneal transplant techniques have not changed much over the past century, lamellar corneal transplant techniques have evolved rapidly. To novices, the numerous acronyms that accompany the various corneal transplant techniques can easily become a disorienting alphabet soup. This article aims to introduce readers to the keratoplasty techniques that are most commonly used today (Figure 1).
When Eduard Konrad Zirm performed the first successful full thickness penetrating keratoplasty in a human in 1905, he became the first person to perform a solid organ transplant. Ironically, he performed the surgery for one of the most challenging indications in ophthalmology – bilateral alkali burns (1-3). His donor was an 11-year-old boy whose eye was enucleated due to foreign body penetration and scleral injury. Emulating Zirm's technique, surgeons began to perform corneal grafting over the subsequent 30 years using enucleated eyes of living donors (4). Vladomir Petrovich Filatov, a Russian ophthalmologist, became known for his work on eye banking in the early 1900s. He suggested using cadaver corneas as donor tissue and developed a method to do so (4).
Over the past century, keratoplasty techniques have evolved considerably. There were early efforts to devise selective tissue replacement techniques that might preserve healthy corneal tissue and avoid risks associated with full-thickness grafting. Anton Elschnig performed the first anterior lamellar keratoplasty in 1914, for a case of interstitial keratitis. Charles Tillet performed the first successful endothelial keratoplasty (EK) case in 1956 for corneal edema. However, the introduction of lamellar techniques actually propelled penetrating keratoplasty (PK) to the forefront of popularity after 1950 (1). Initially, anterior lamellar techniques were fraught with the problems of interface haze, scarring, and epithelial ingrowth. Tillet's EK technique, although successful, was not repeated and no additional clinical cases were reported for decades.
It was not until the late 1990s that EK was reinvestigated, revised, and reintroduced into clinical practice, launching the modern era of lamellar keratoplasty. Gerrit Melles experimented with eye bank cadaver eyes and then with animal eyes to bring EK into the modern era. Melles described an approach called posterior lamellar keratoplasty (PLK), in which the posterior cornea was dissected out and replaced with posterior stroma and endothelium from donor corneal tissue (1, 5-7) . Melles contributed the foundational concept of self-adherent graft tissue that required no sutures and could be supported initially by an air bubble. In 1999, Mark Terry introduced modifications to simplify Melles' PLK technique, developed new instrumentation, and coined the technique deep lamellar endothelial keratoplasty (DLEK) (1, 8) . However, these techniques were technically difficult to perform, required extensive manual lamellar dissection, and were not adopted widely. Patients healed rapidly compared to full-thickness transplants, but the presence of a deep stroma-to-stroma interface limited postoperative visual acuity typically to the 20/40-20/50 range.
In 2004, Melles made additional technical modifications, and introduced the idea of stripping and removing the patient's Descemet membrane and endothelium with his Descemetorhexis technique. This new technique was renamed Descemet stripping endothelial keratoplasty (DSEK). After Mark Gorovoy introduced the microkeratome for automated preparation of donor cornea, manual lamellar dissection could be eliminated entirely, and the procedure was again renamed as Descemet stripping automated endothelial keratoplasty (DSAEK). Francis Price proposed additional technical modifications, and again, Terry introduced simplifications and new instrumentation. DSAEK allowed patients to achieve improved postoperative visual acuity results, to the 20/25-20/30 range, because its graft-host interface is more smooth (1, 9) . With the advent of eye bank prepared donor tissue in 2006, financial and technical obstacles were removed, and DSAEK surgery became the most commonly performed method of endothelial keratoplasty and procedure of choice for the treatment of corneal edema.
In 2006, Melles went on to describe a technique known as Descemet membrane endothelial keratoplasty (DMEK) that allowed for transplantation of a pure Descemet membrane and endothelium graft, and exact anatomical replacement of diseased tissue in cases of endothelial dysfunction. Compared to DSAEK, DMEK allows even faster visual recovery, better postoperative visual acuity results, and greater overall patient satisfaction due to elimination of the stroma-to-stroma graft-host interface (10). However, the initial donor preparation failure rate and surgical learning curve prevented widespread application after introduction of this technique (1). Mirroring the evolution of DSAEK, as surgical techniques have become standardized and eye banks have begun to prepare DMEK graft tissue, DMEK is rapidly becoming the procedure of choice for endothelial keratoplasty for the treatment of Fuchs endothelial dystrophy and pseudophakic bullous keratopathy.
Additionally, anterior lamellar keratoplasty (ALK) techniques have been refined over the past 40 years. In the late 1970's Malbran and Gasset were performing deep anterior lamellar keratoplasty (DALK) to excise and replace the corneal tissue anterior to the deepest stromal lamellae with impressive results including 80% of keratoconus patients achieving 20/40 or better visual acuity (1, 11) . However, obstacles remained that limited the popularity of this approach, including achievement of a reproducible separation plane between posterior stroma and, ideally, Descemet membrane. In 2002, Anwar and Teichmann introduced their "big bubble" pneumodissection technique in which a bubble of air is injected deep into the corneal stroma to establish separation of the posterior stroma from Descemet membrane (12). Their technique has allowed surgeons to achieve more consistent results than previous methods, but in some cases, intraoperative conversion to a full-thickness PK is still required. For patients with keratoconus or scarring that does not involve Descemet membrane or endothelium, DALK is considered by most to be the surgical treatment of choice (1), although extended operating times due to the need for careful lamellar dissection have limited its popularity.
Keratoprosthesis, the transplantation of an artificial cornea, was first performed in Italy by Benedetto Strampelli the 1960s (1). Patients requiring repeat corneal transplantation highlighted the need for an alternative to corneal allograft treatment, as graft survival rates drop with each additional procedure. Historical options have included the osteo-odonto-keratoprosthesis (OOKP) and AlphaCor artificial cornea. These have since been largely replaced by the Boston Type I Keratoprosthesis (KPro), which became approved for use by the U.S. Food and Drug Administration in 1992 (1, 13). The device consists of a clear plastic optic and a prosthetic plate that are sandwiched around a donor allograft or the patient's own corneal tissue. The device is then sutured onto the recipient eye to replace a failed graft or the native cornea. Keratoprosthesis surgery is a procedure of last-resort, reserved for patients who are not candidates for other types of keratoplasty.
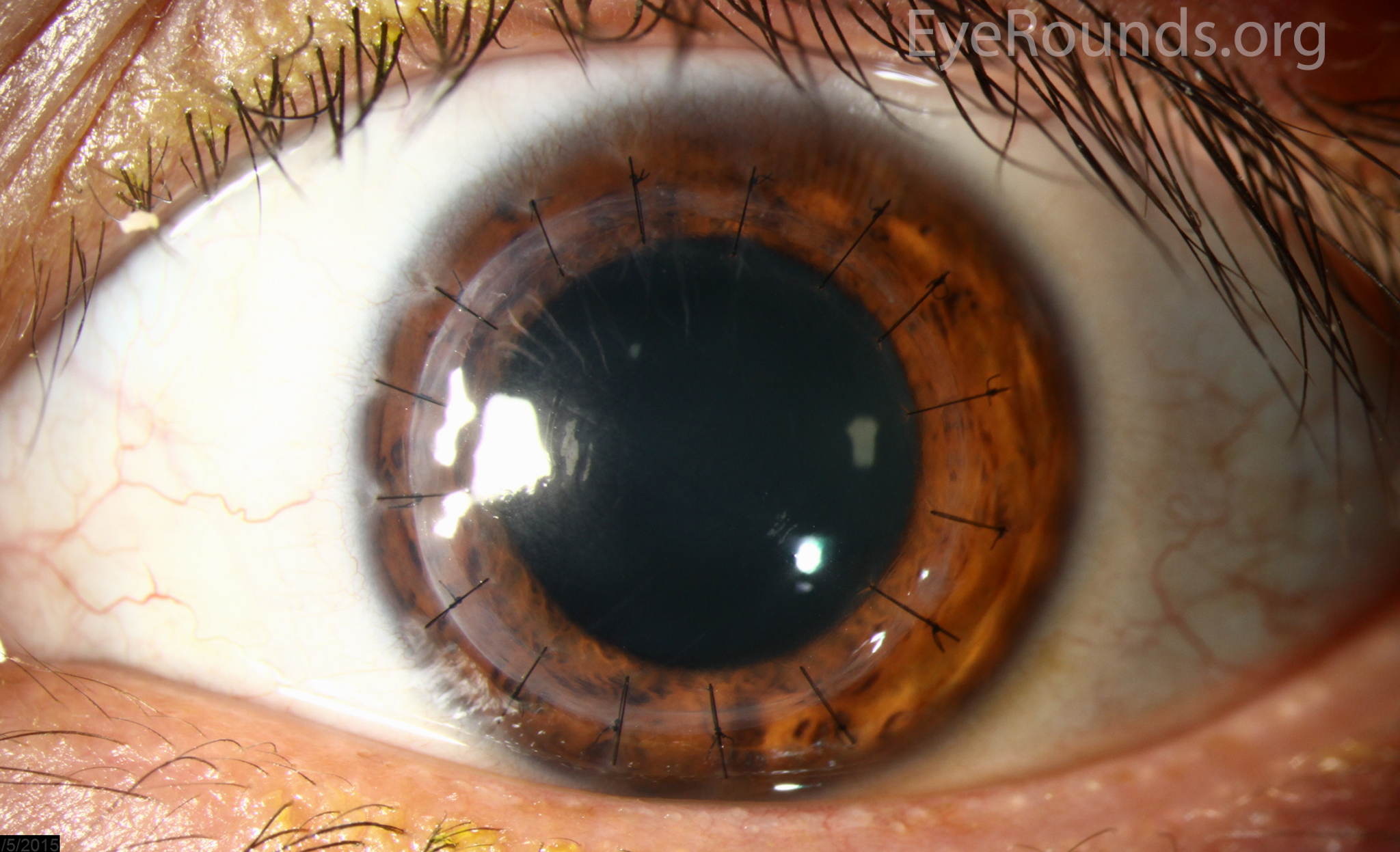
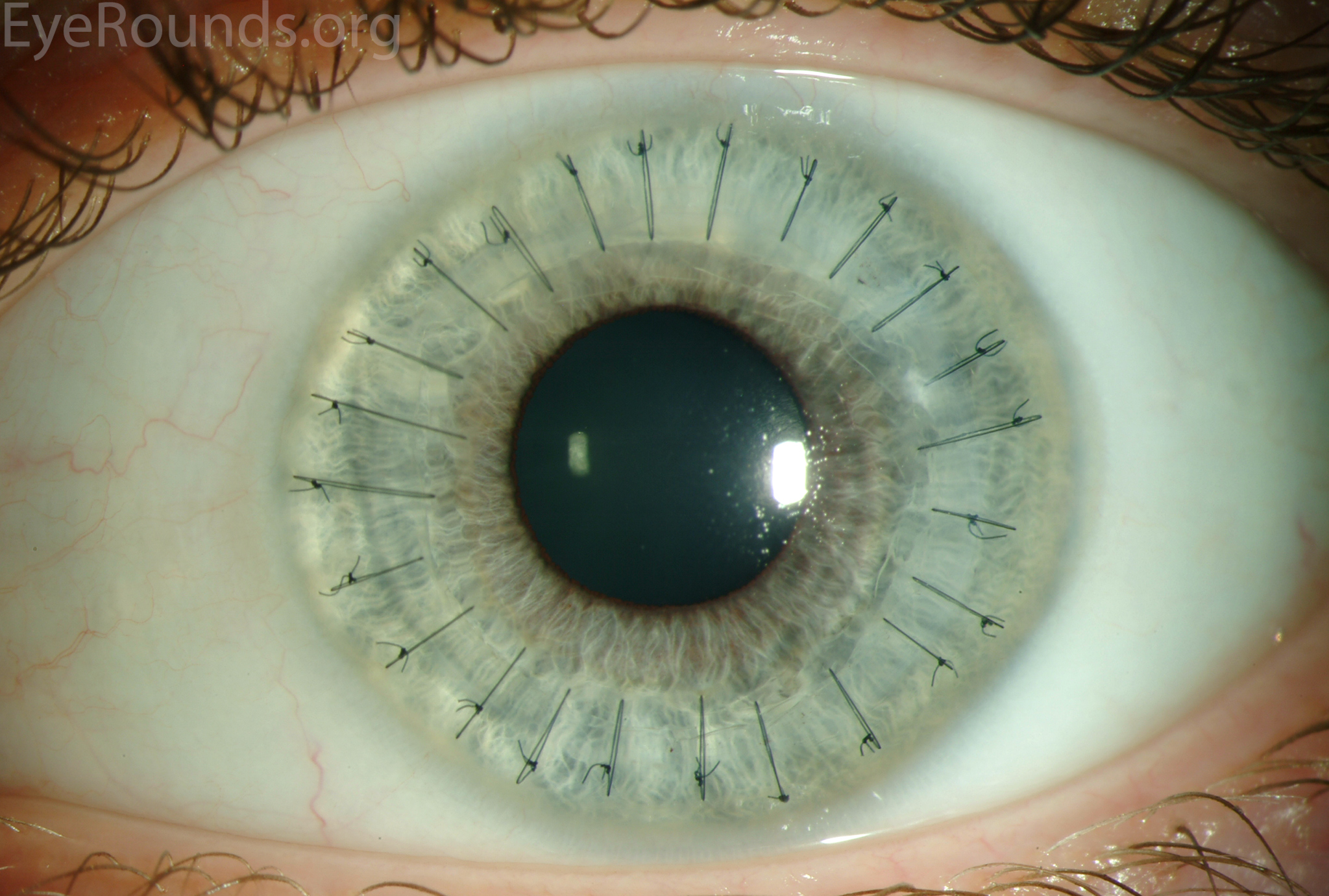
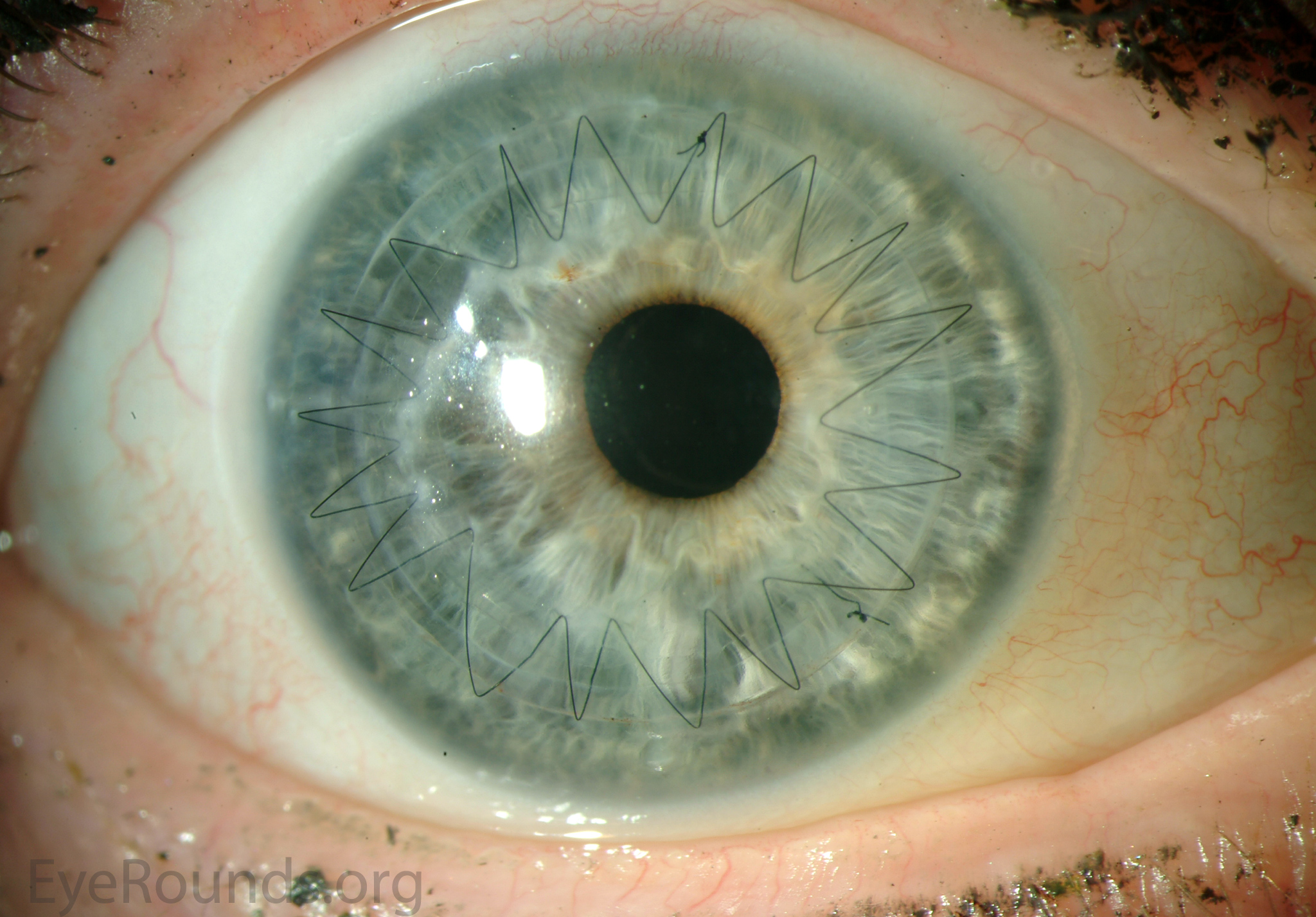
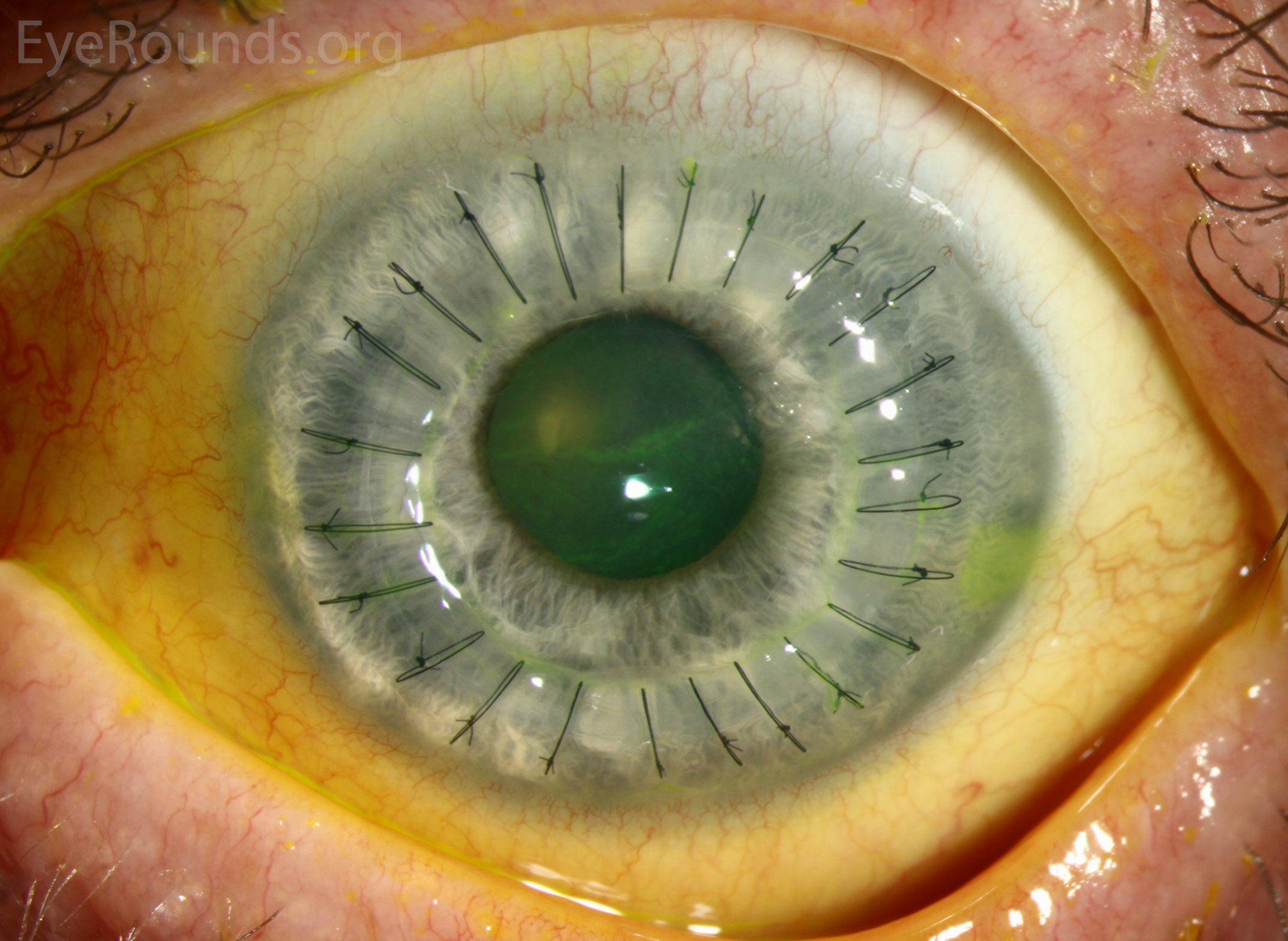
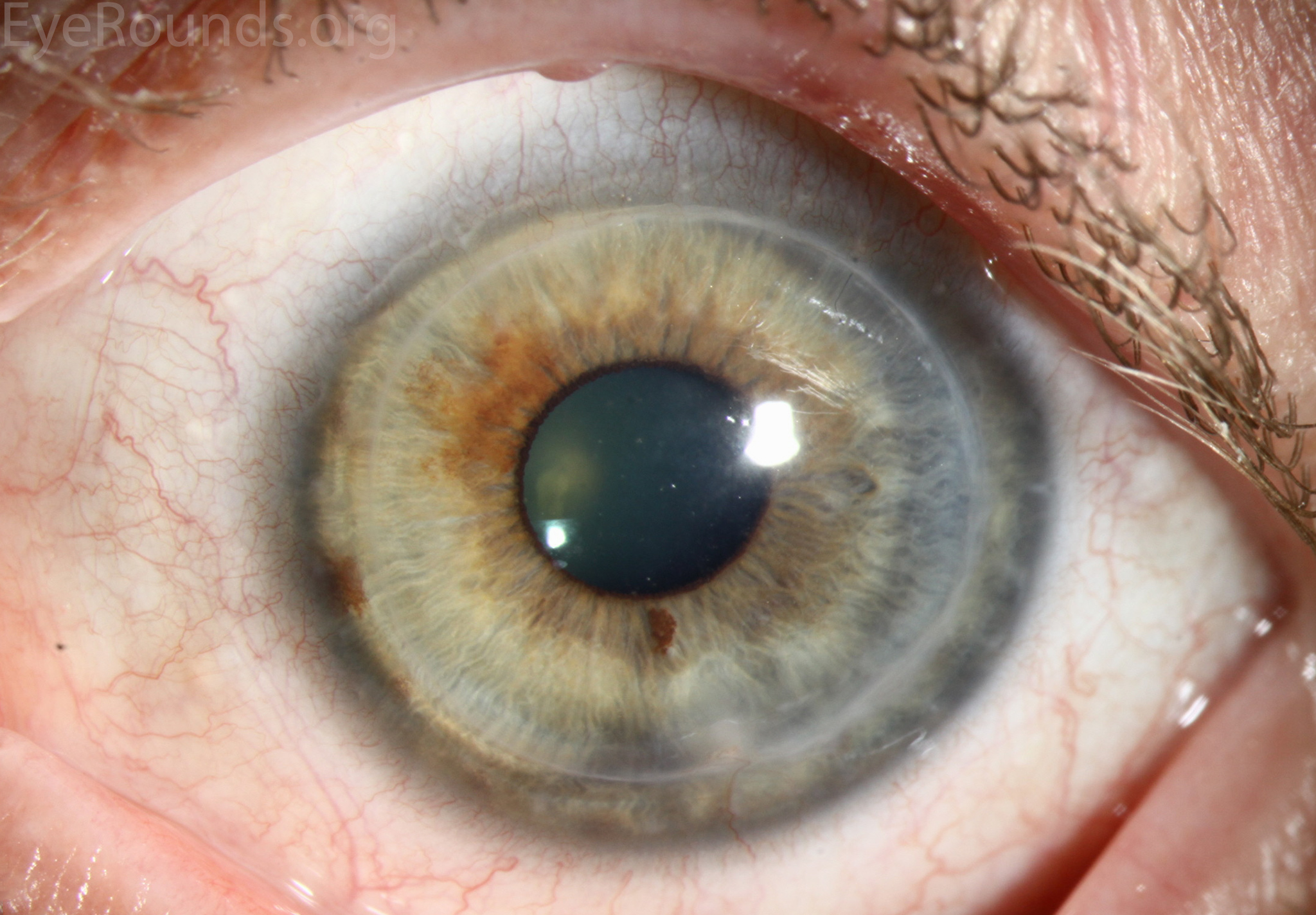
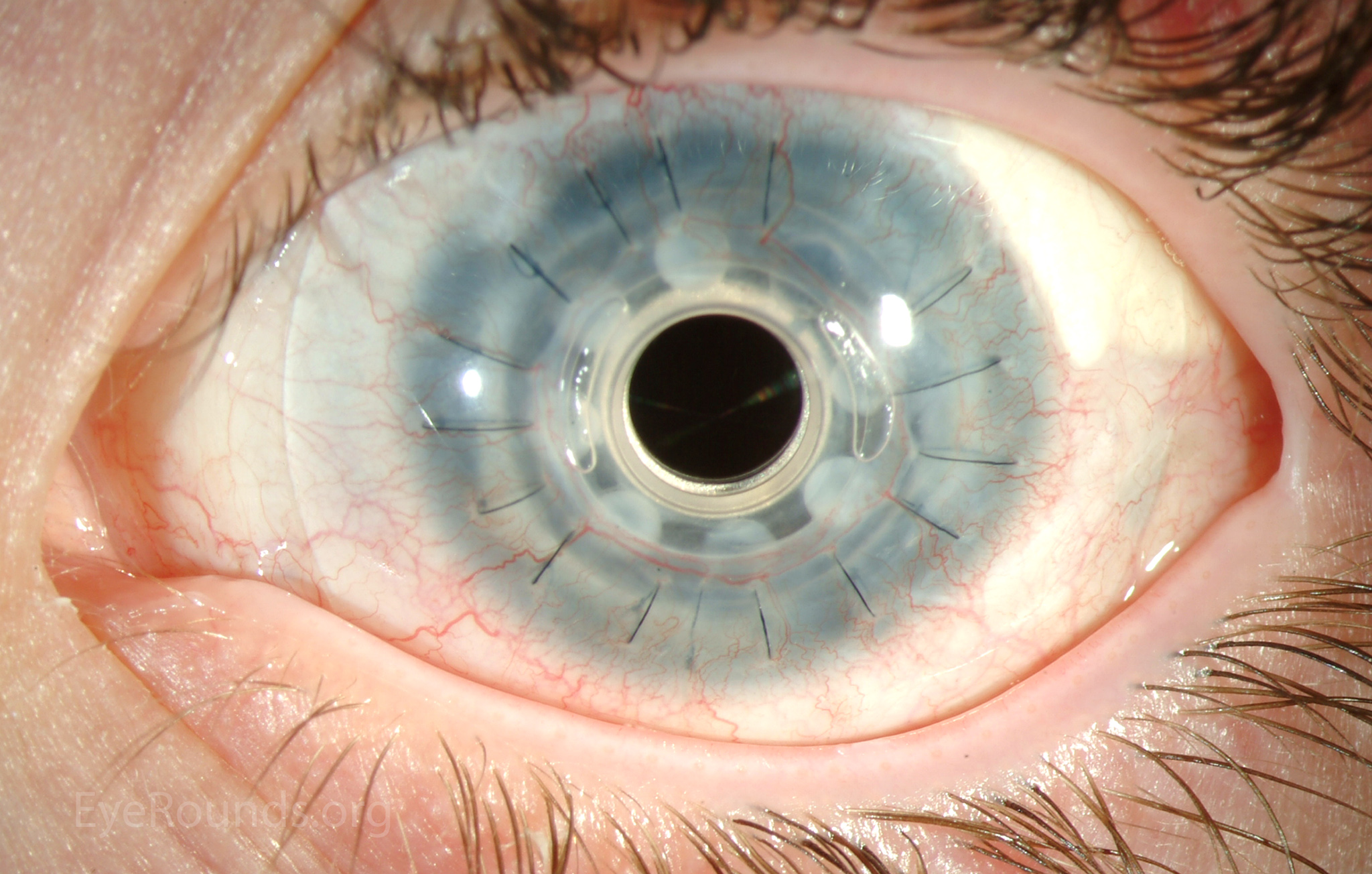
PK is a full-thickness transplant procedure, in which a trephine of an appropriate diameter is used to make a full-thickness resection of the patient's cornea, followed by placement of a full-thickness donor corneal graft. Interrupted and/or running sutures are placed in radial fashion at equal tension to minimize post-operative astigmatism (Figure 2). Later, the sutures are removed selectively to reduce the amount of astigmatism present. A transplant can last decades with proper care (Figure 3). While once the most prominent type of corneal transplant, PK has been supplanted by partial thickness techniques for endothelial dysfunction without significant stromal scarring. PKs are performed primarily for visually significant stromal scarring, opacities with an uncertain status of the endothelium or significant posterior corneal involvement, corneal ectasia (such as keratoconus and pellucid marginal degeneration, especially if there is history of hydrops), combined stromal and epithelial disease (such as Peters anomaly), and infectious or non-infectious corneal ulcerations or perforations (1, 14). A variant of the procedure, the mini-PK, can be used to treat more focal defects in the cornea (Figure 4).
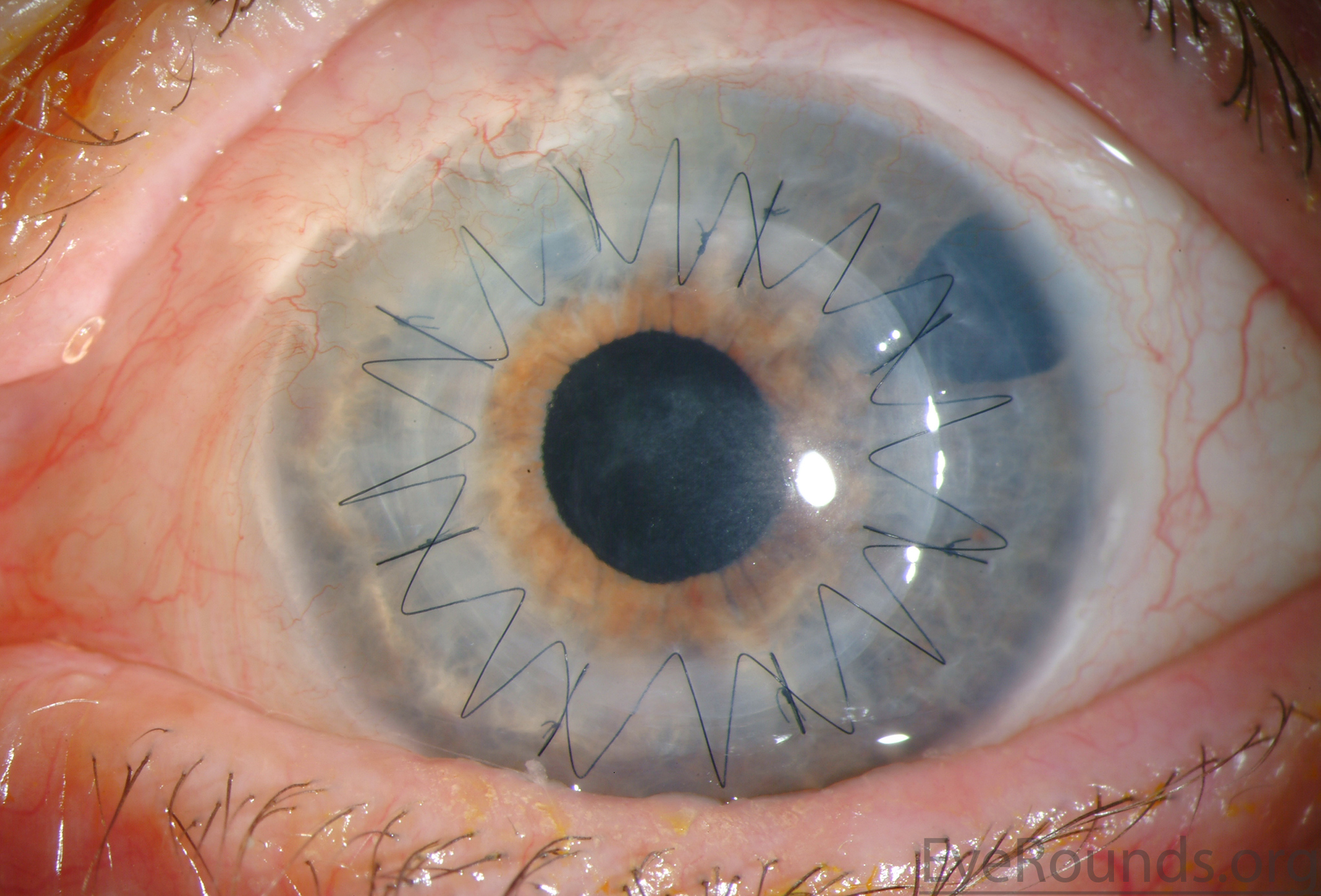
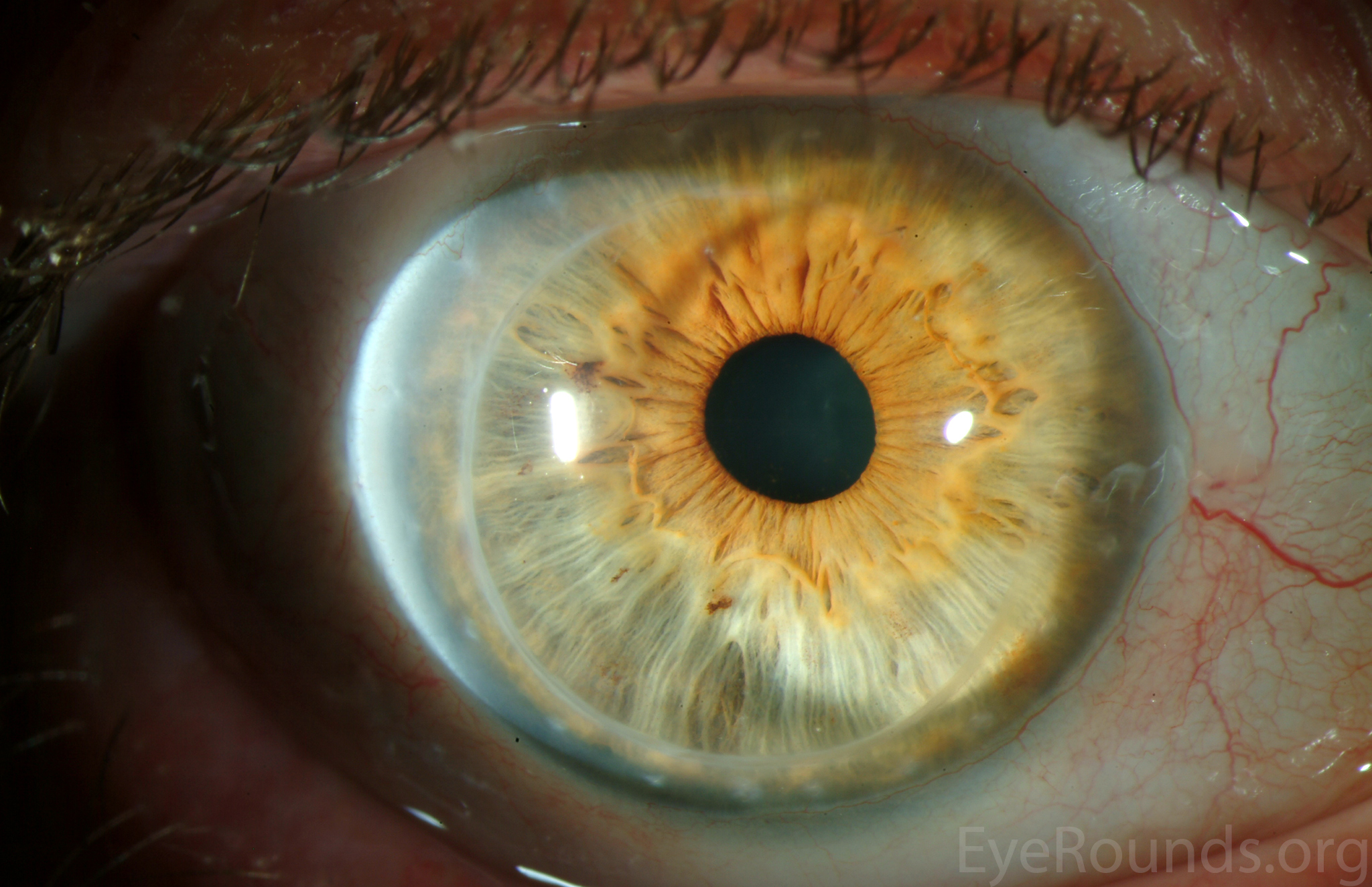
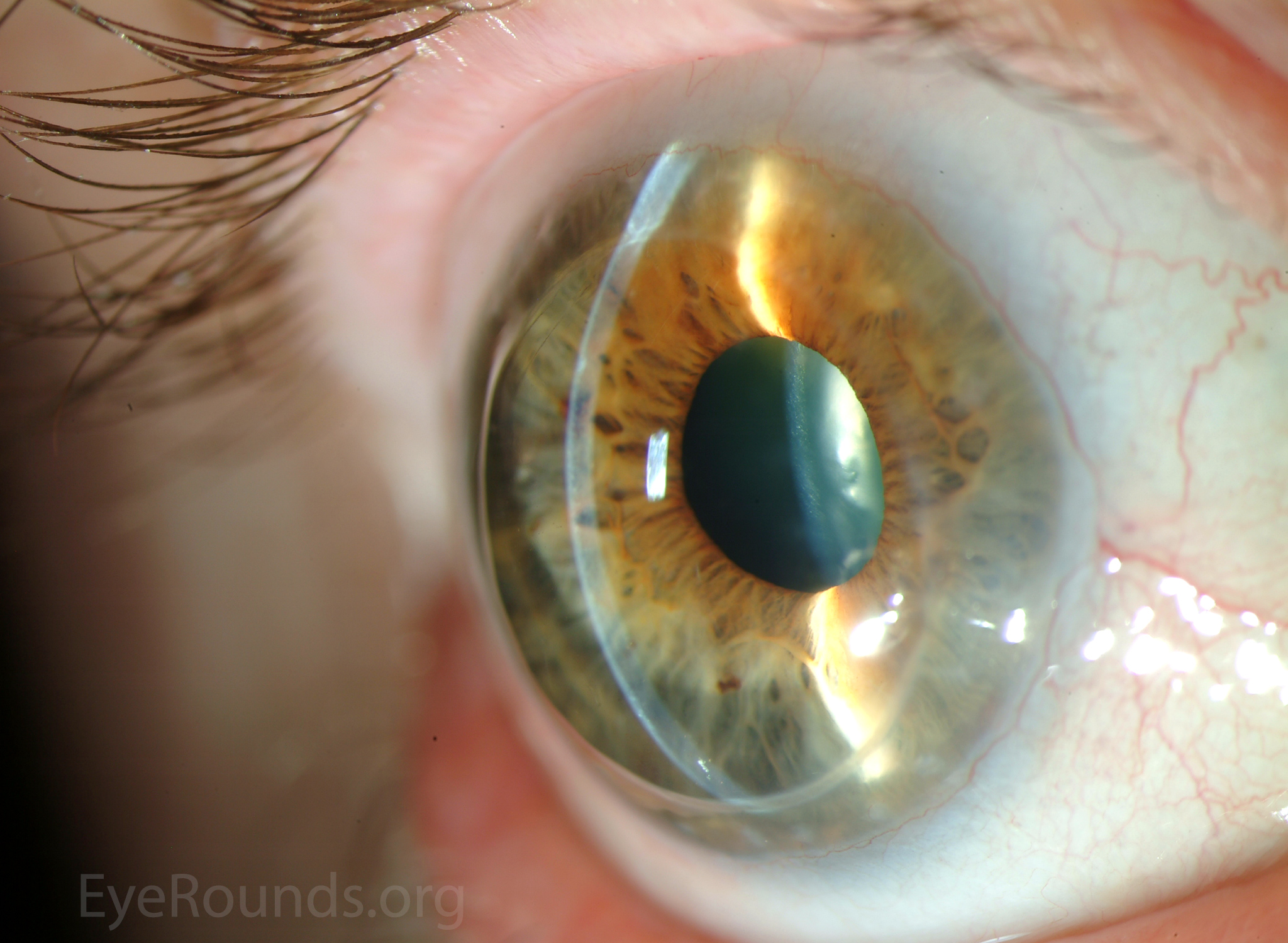
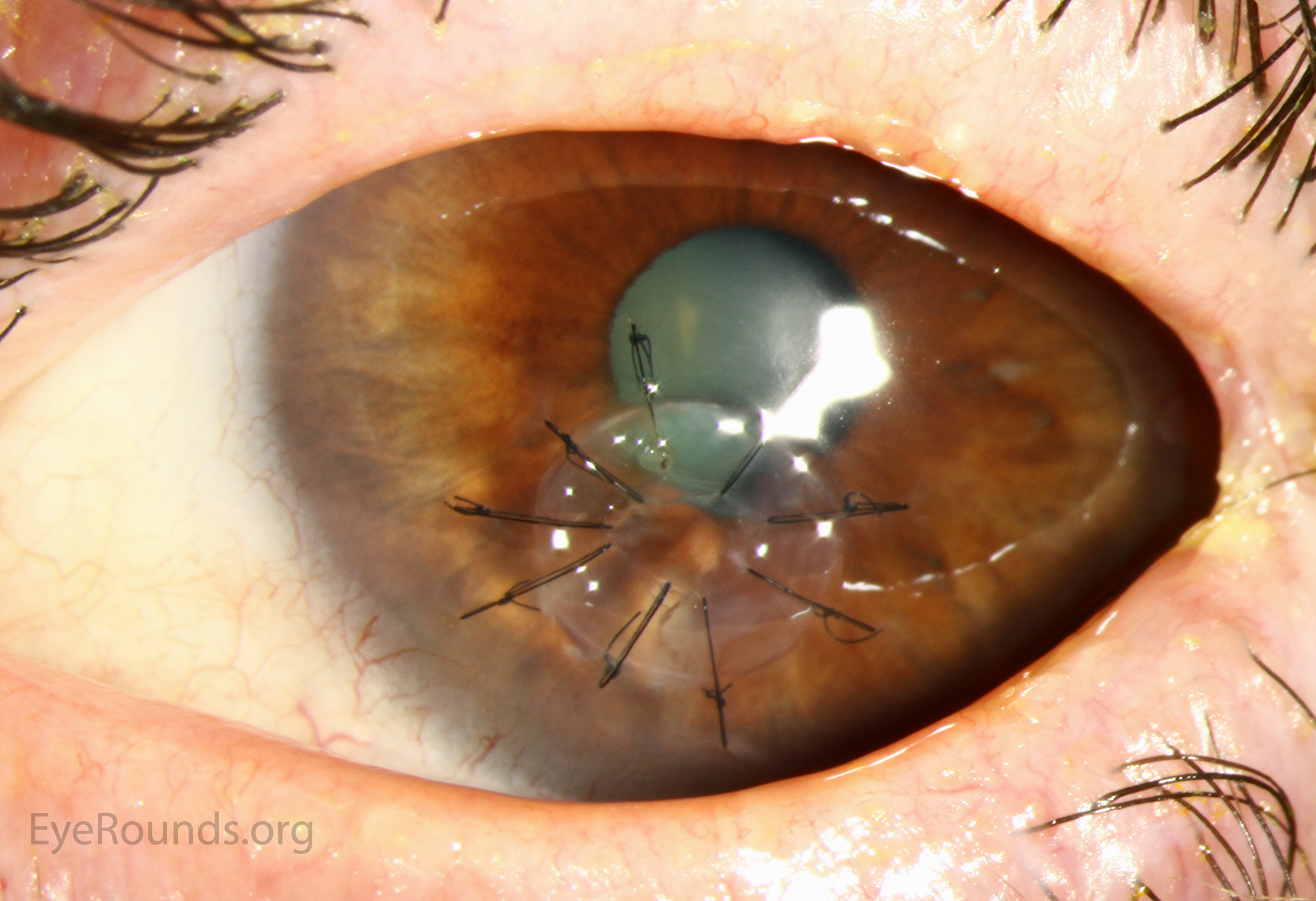
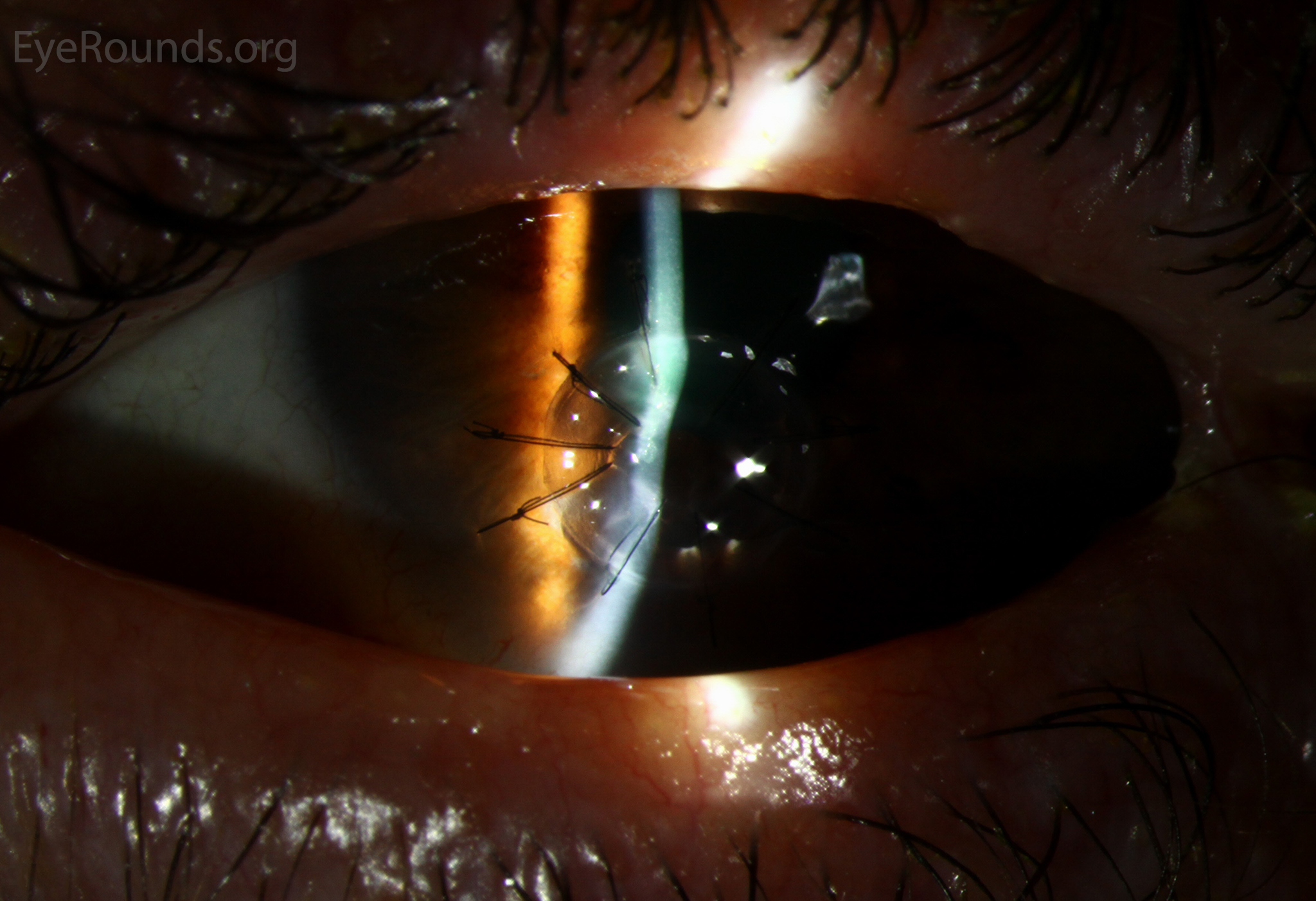
PK grants the ability to treat disease in the epithelial, stromal, and endothelial layers. A full-thickness graft also eliminates optical interface related visual problems that may exist with lamellar transplants with a stroma-stroma interface.
However, postoperative recovery time is relatively long, sometimes taking years to achieve best-corrected visual acuity. Frequently, there is substantial postoperative refractive error due to high regular or irregular astigmatism of the graft, and a higher chance of requiring rigid gas permeable contact lens wear to correct astigmatic error. There is a higher risk of allograft rejection compared with other keratoplasty types. Additionally, PKs carry a higher lifetime risk of wound dehiscence due to the compromised tectonic strength that comes from a full-thickness wound.
Video 1: PK in a patient with severe corneal scarring after bacterial keratitis in the setting of HSV-related neurotrophic disease. Video contributed by Jesse Vislisel, MD If video fails to load, use this link.
Basic procedure steps (Video 1)
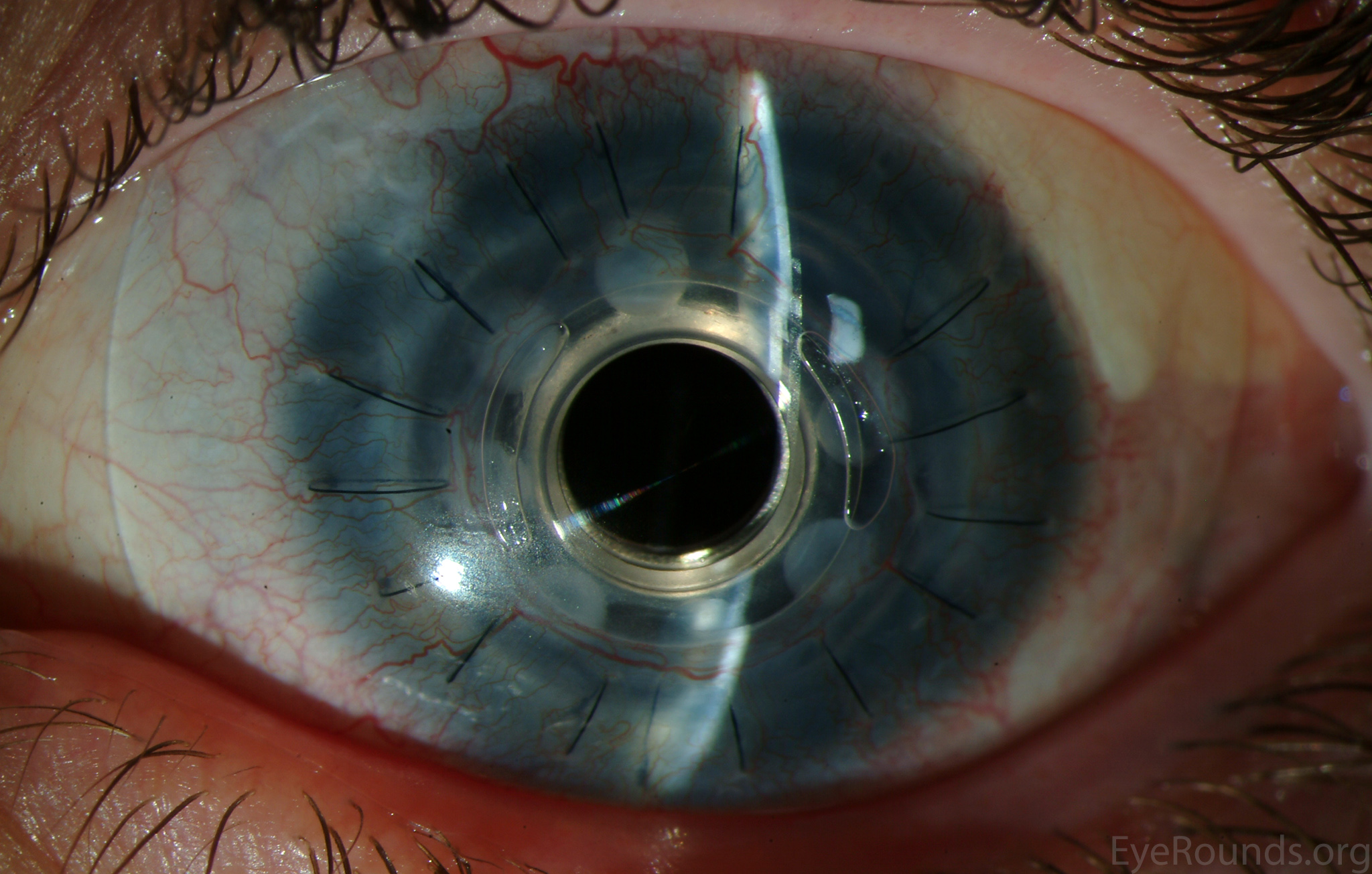
DALK is a partial-thickness cornea transplant procedure that involves selective transplantation of the corneal stroma, leaving the native Descemet membrane and endothelium in place. A trephine of an appropriate diameter is used to make a partial-thickness incision into the patient's cornea, followed by pneumodissection or manual dissection of the anterior stroma. This is followed by placement of a graft prepared from a full-thickness punch in which the donor endothelium-Descemet membrane complex has been removed. The intention is to preserve the patient's Descemet membrane and endothelium. Similar to PK, the graft is secured with interrupted and/or running sutures (Figure 5) and these are then selectively removed post-operatively (Figure 6).
DALK is useful for processes involving the corneal stroma in the presence of healthy endothelium. Examples include corneal ectasia (such as keratoconus in the absence of hydrops), corneal scars that are not full-thickness, and corneal stromal dystrophies (1, 15, 16).
Because it is not a full-thickness procedure, the resultant wound is stronger than that of a PK. Leaving the host endothelium intact significantly decreases the risk of endothelial rejection.
The surgery is more complex and difficult to perform than PK. If the Descemet membrane is perforated intraoperatively, the surgeon must convert to a PK. The "big bubble" technique makes dissection more consistent and is the preferred technique at our institution (12).
Video 2: Big bubble DALK technique. Video contributed by Matt Ward, MD and Mark Greiner, MD -- If video fails to load, use this link
Additional Resources:
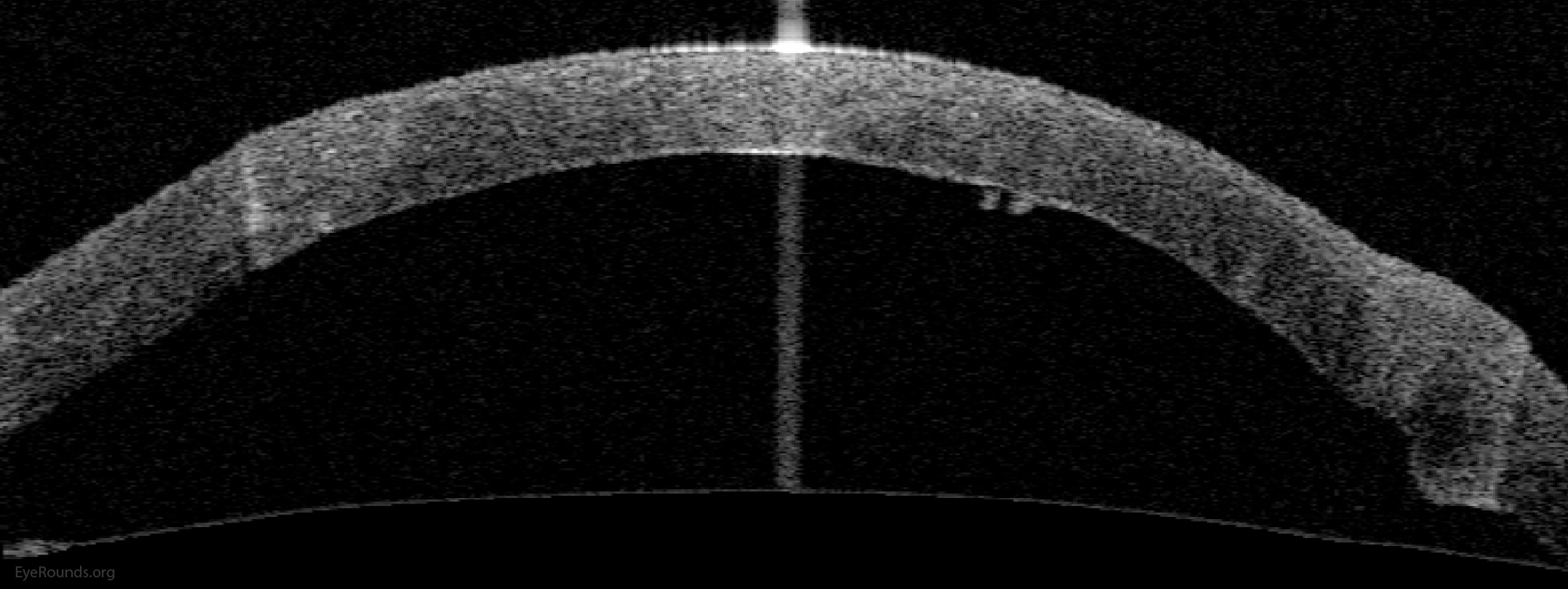
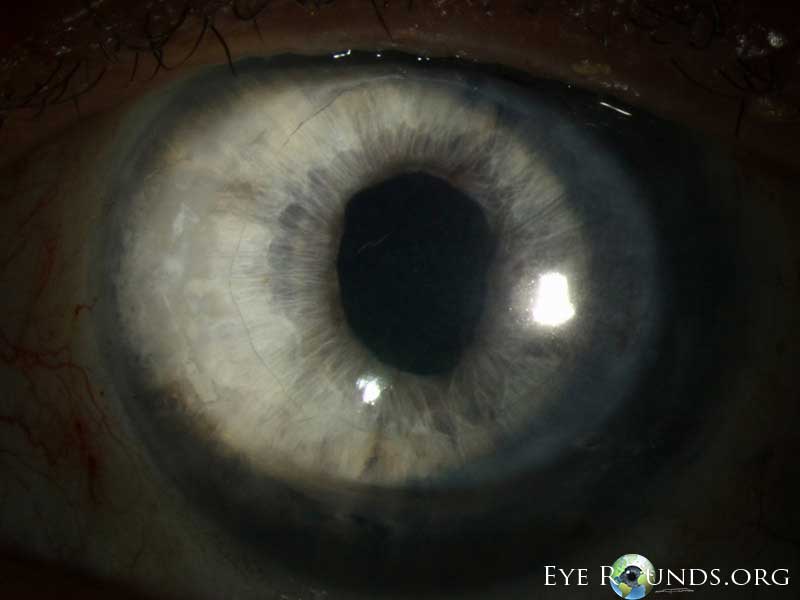
Video Links ArticlesDSAEK is a partial thickness cornea transplant procedure that involves selective removal of the patient's Descemet membrane and endothelium, followed by transplantation of donor corneal endothelium in addition to donor corneal stroma (Figure 8). The transplanted tissue is approximately 100-200 microns thick. If the endothelium of the graft makes contact with any surgical instruments, it will be damaged and the graft may fail; therefore, the surgical procedure is designed to avoid contacting the donor endothelium. A tunneled corneoscleral incision is created, the recipient endothelium and Descemet membrane is removed, the graft is folded and inserted with non-coapting forceps (forceps that do not meet at the tips), and an air bubble is placed in the anterior chamber to support graft adherence. The procedure is used to treat corneal edema in the setting of endothelial dystrophies (such as Fuchs corneal dystrophy and posterior polymorphous corneal dystrophy), pseudophakic bullous keratopathy, iridocorneal endothelial (ICE) syndrome, endothelial failure in the setting of prior intraocular surgery or of a previous PK graft, and other causes of corneal endothelial dysfunction (1, 17-20).
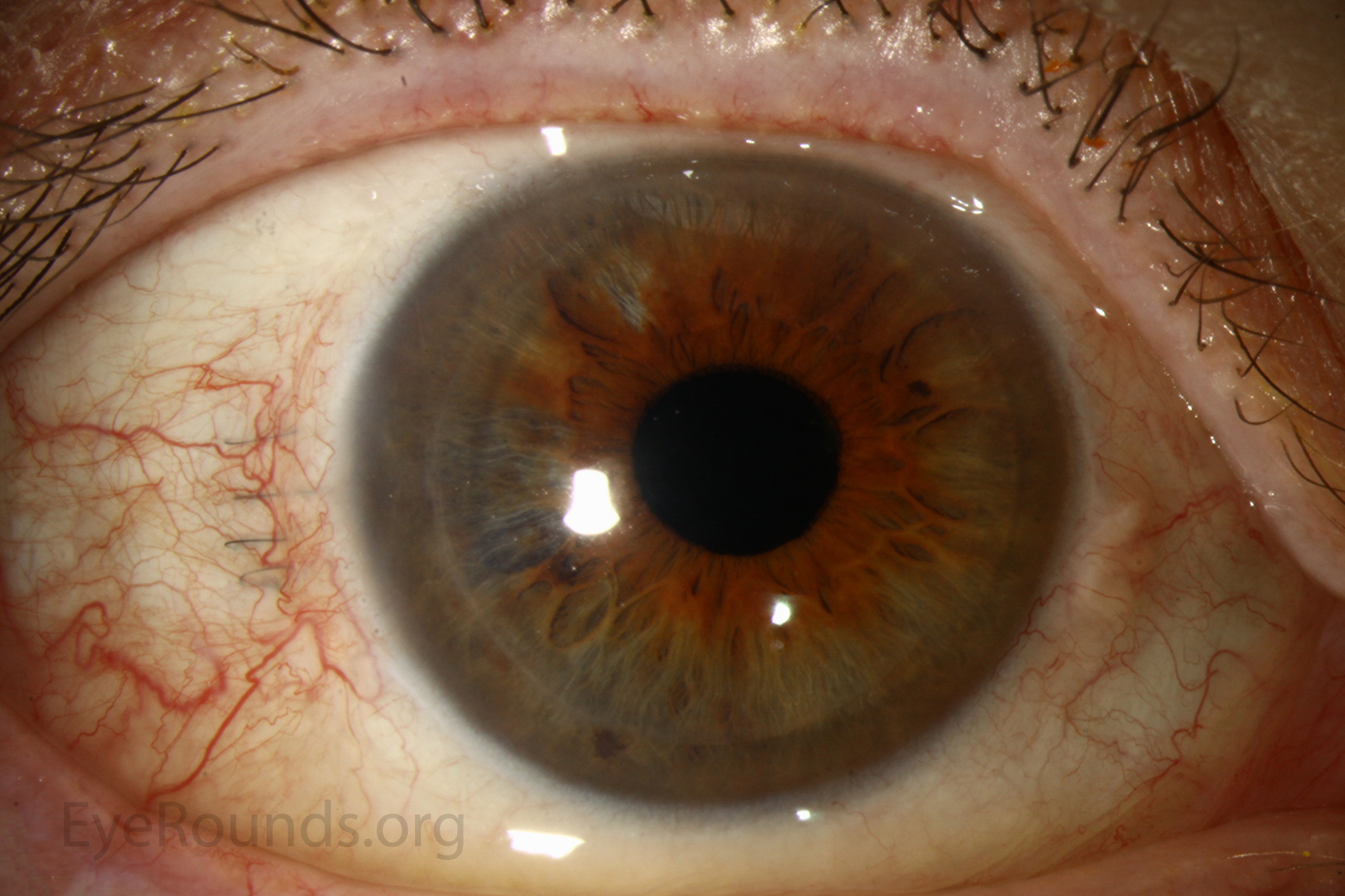
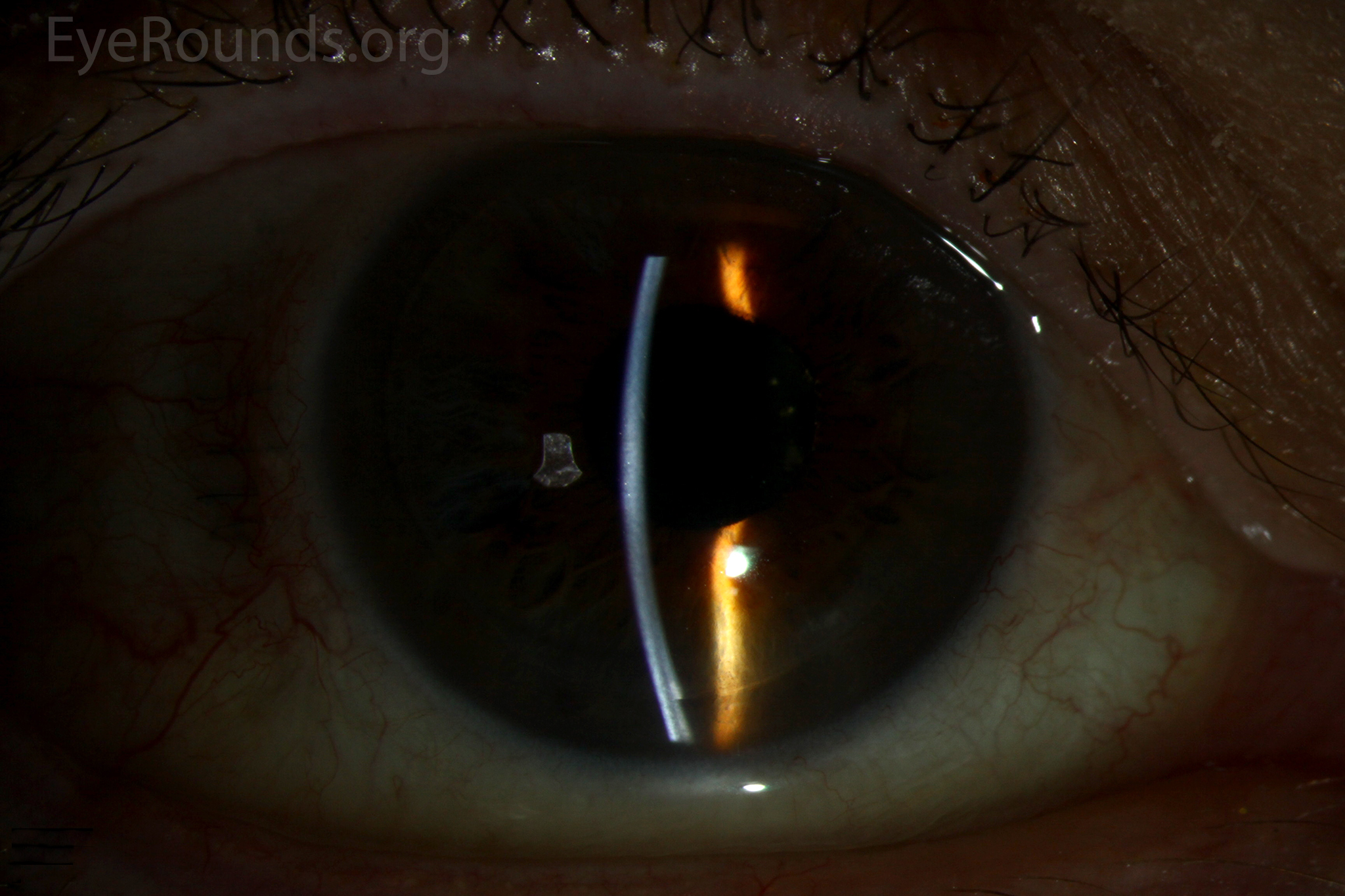
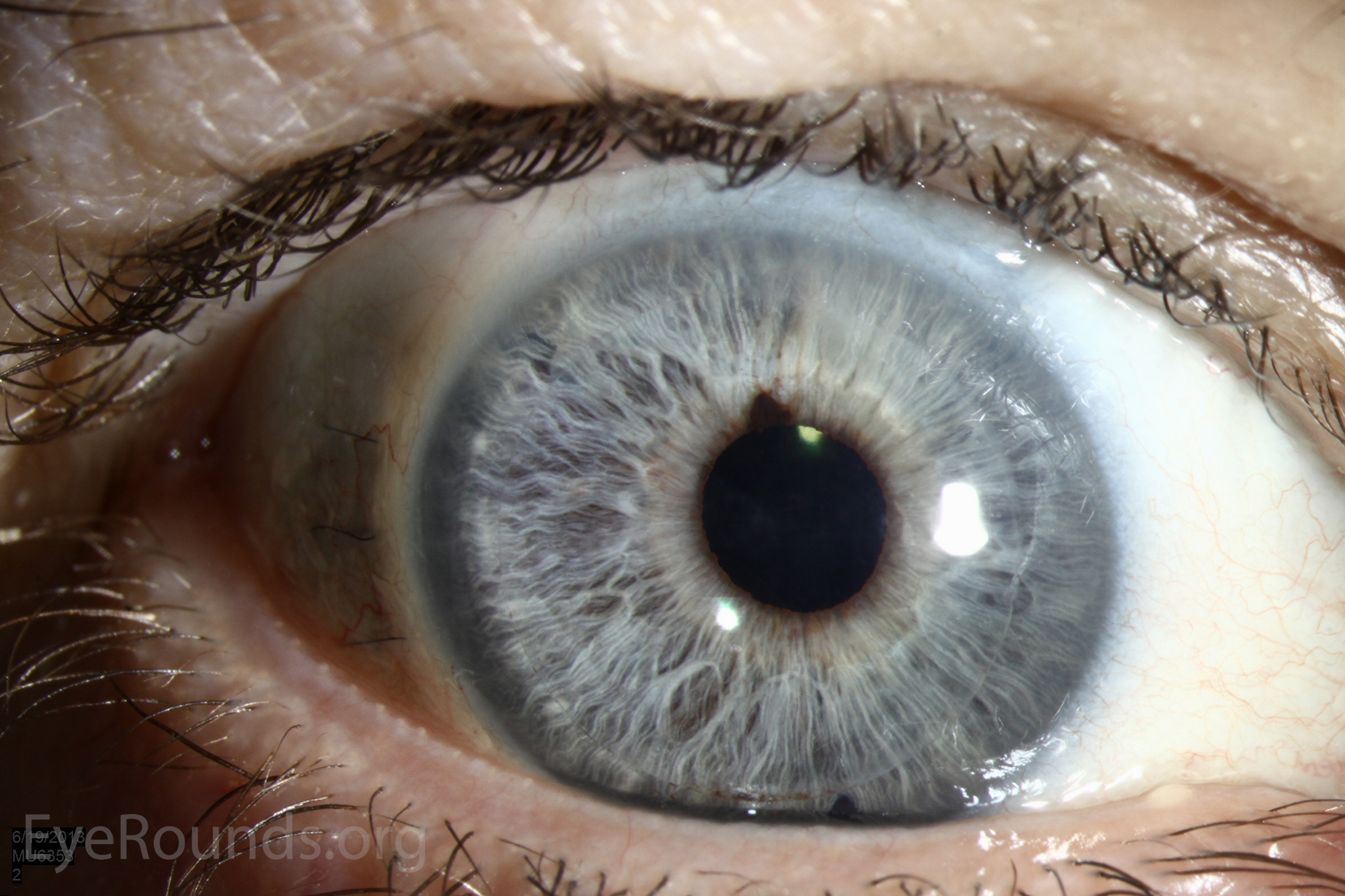
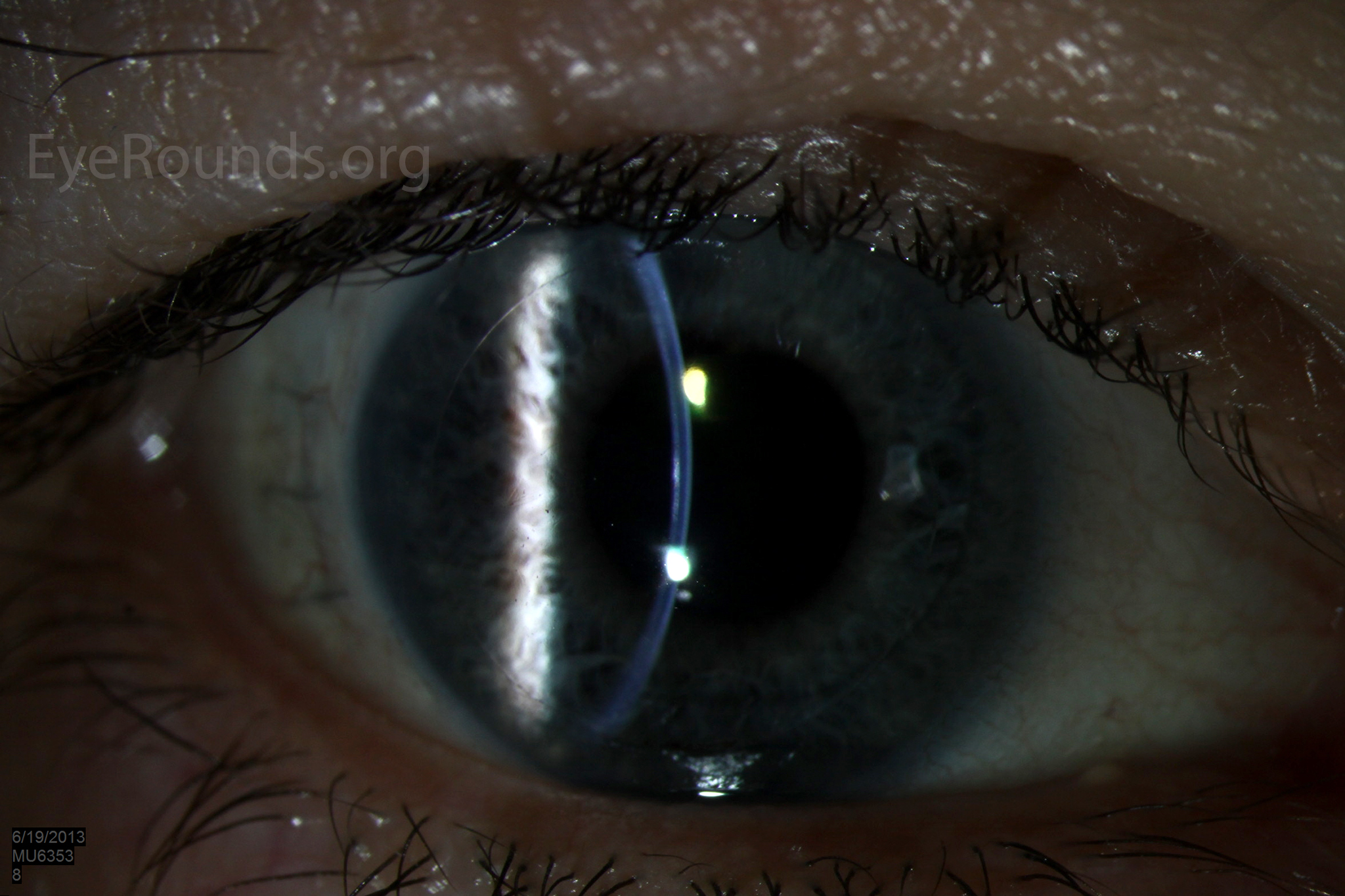
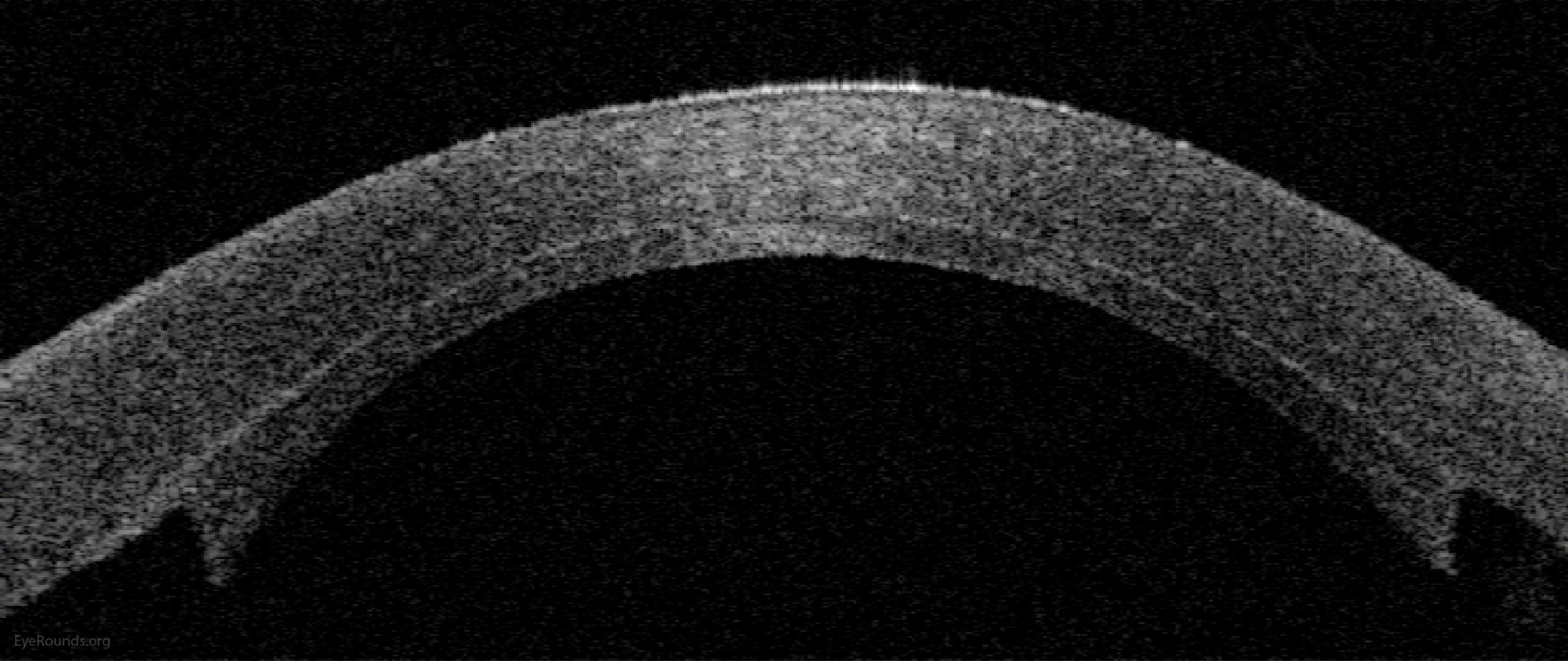
DSAEK offers the advantage of relatively rapid healing time and visual rehabilitation. Compared to PK and DALK, there is less risk of graft rejection and suture-related complications. There is minimal topographic change to the corneal curvature. A somewhat predictable hyperopic shift results (typically 0.8-1.5 D), making intraocular lens selection easier when performing staged or simultaneous cataract surgery.
Postoperative visual acuity can be very good, but there is some limitation from the effects of the stroma-to-stroma graft-host interface. There is also a risk of postoperative graft dislocation.
Video 3: Descemet stripping automated endothelial keratoplasty (DSAEK) is an endothelial replacement procedure in which dysfunctional corneal endothelium is replaced with a graft consisting of donor endothelium and a thin layer of posterior stroma to facilitate handling of the tissue. This case was performed for severe corneal edema secondary to pseudophakic bullous keratopathy. Contributed by Jesse Vislisel, MD, and Mark A. Greiner, MD
Basic procedure steps (Video 3):
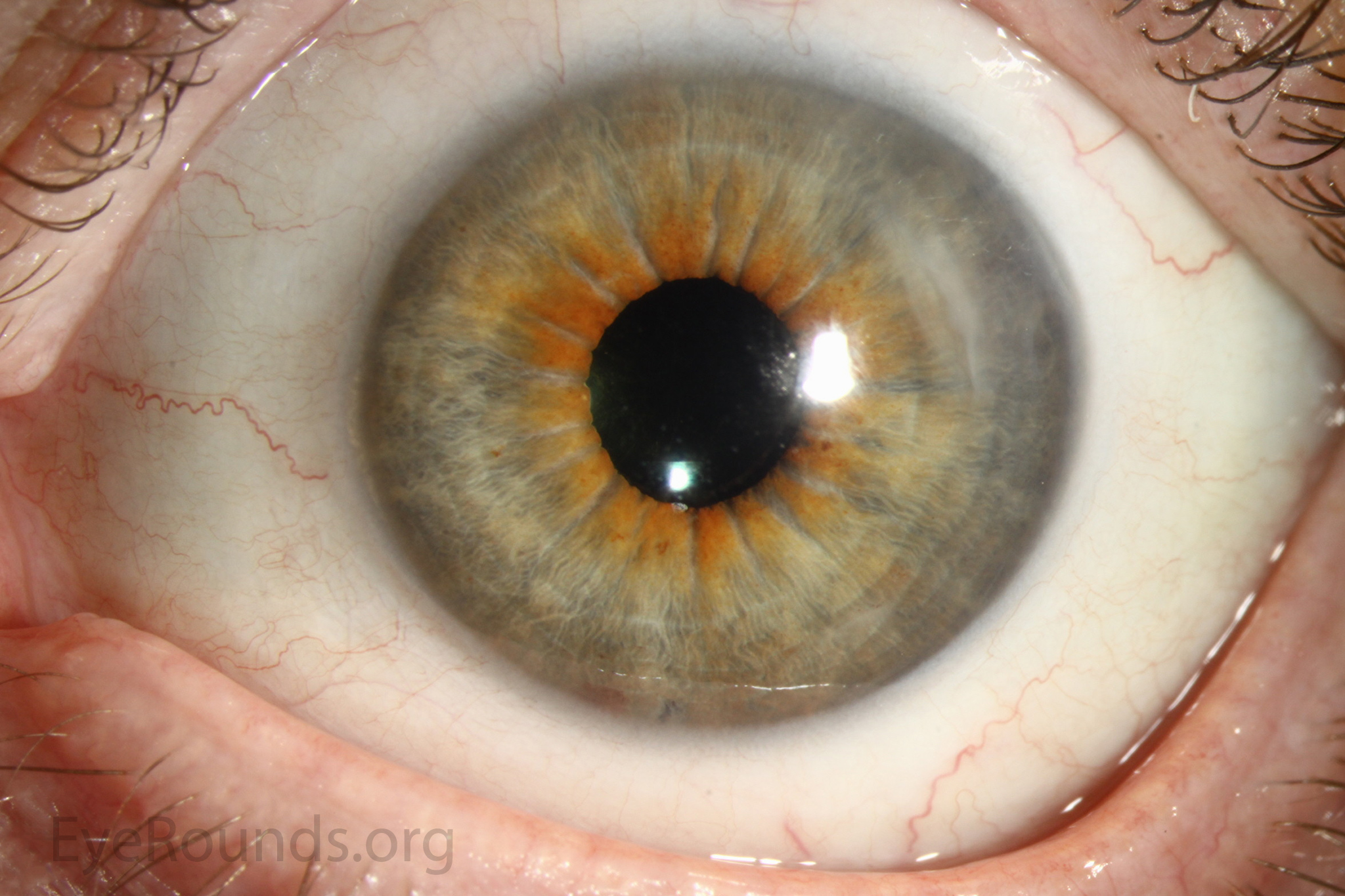
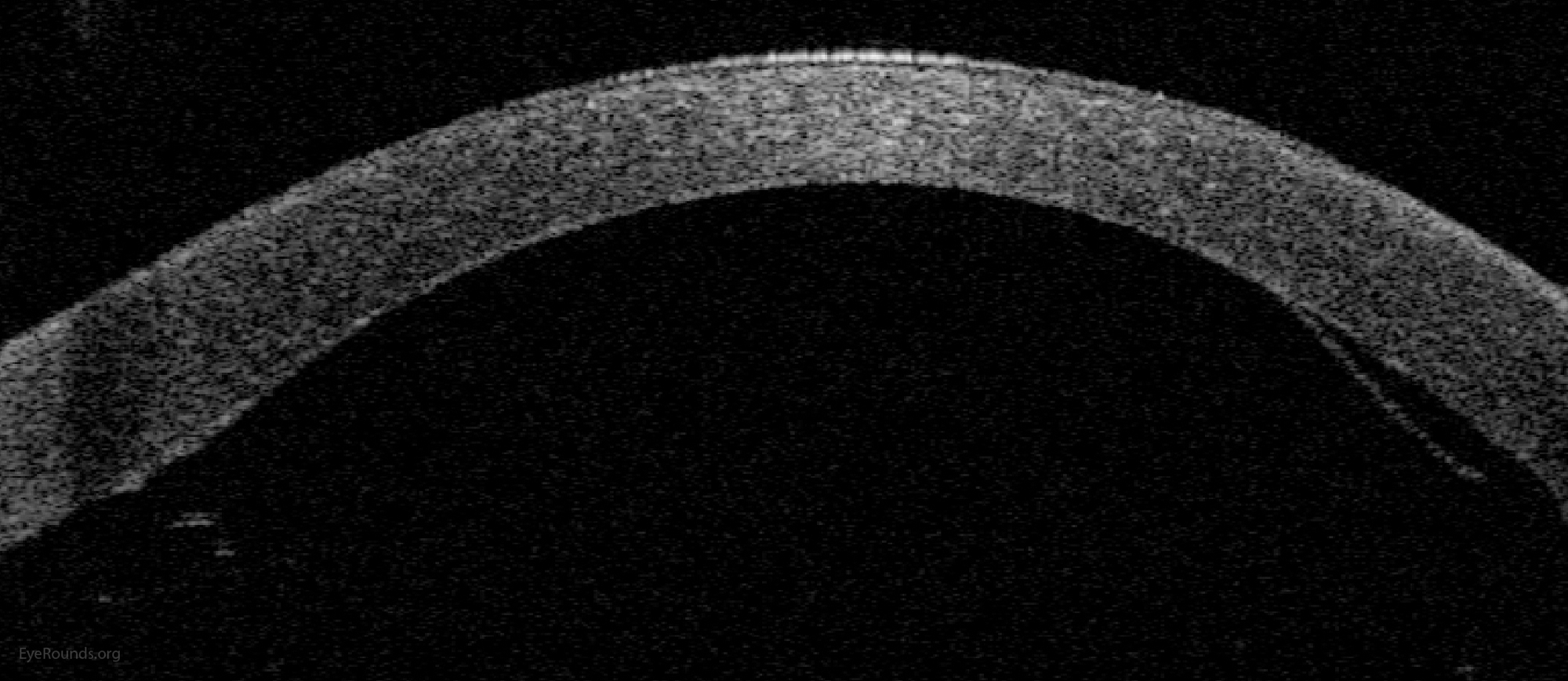
DMEK is a partial-thickness cornea transplant procedure that involves selective removal of the patient's Descemet membrane and endothelium, followed by transplantation of donor corneal endothelium and Descemet membrane without additional stromal tissue from the donor. The graft tissue is merely 10-15 microns thick. Similar to DSAEK, direct contact with the DMEK graft tissue should be avoided to prevent endothelial cell damage and graft failure. A clear corneal incision is created, the recipient endothelium and Descemet membrane are removed, and the graft is loaded into an inserter. After injecting the tissue into the anterior chamber, the surgeon orients and unscrolls the graft, and a bubble of 20% sulfur hexafluoride (SF6) is placed in the anterior chamber to support graft adherence (Figure 10). A variation known as Descemet membrane automated endothelial keratoplasty (DMAEK) utilized an automated preparation of the donor tissue that left a rim of donor stroma peripherally for easier tissue handling (Figure 11), but the procedure is no longer performed due to advances in DMEK that have allowed for easier insertion and manipulation of the graft tissue.
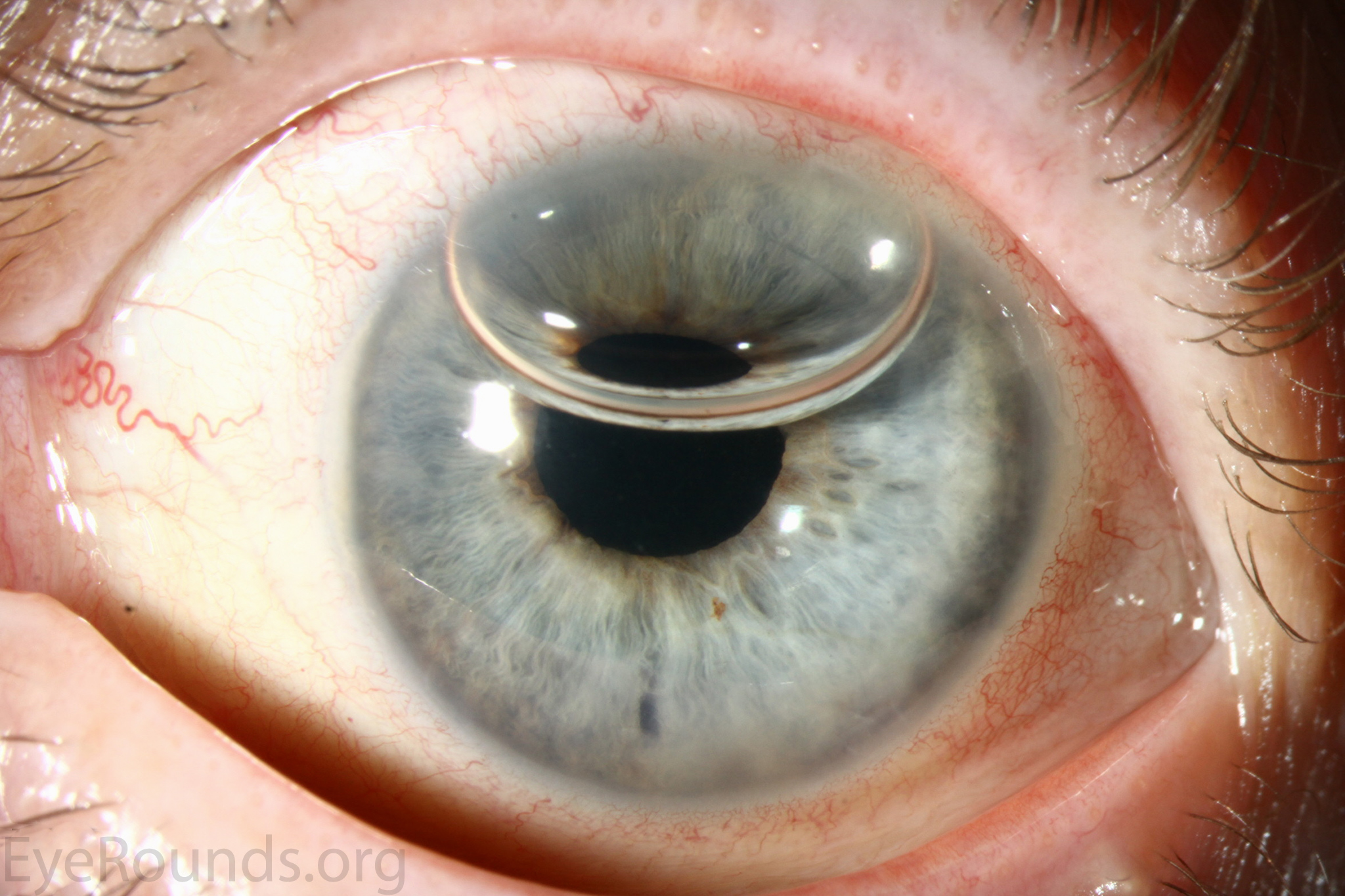
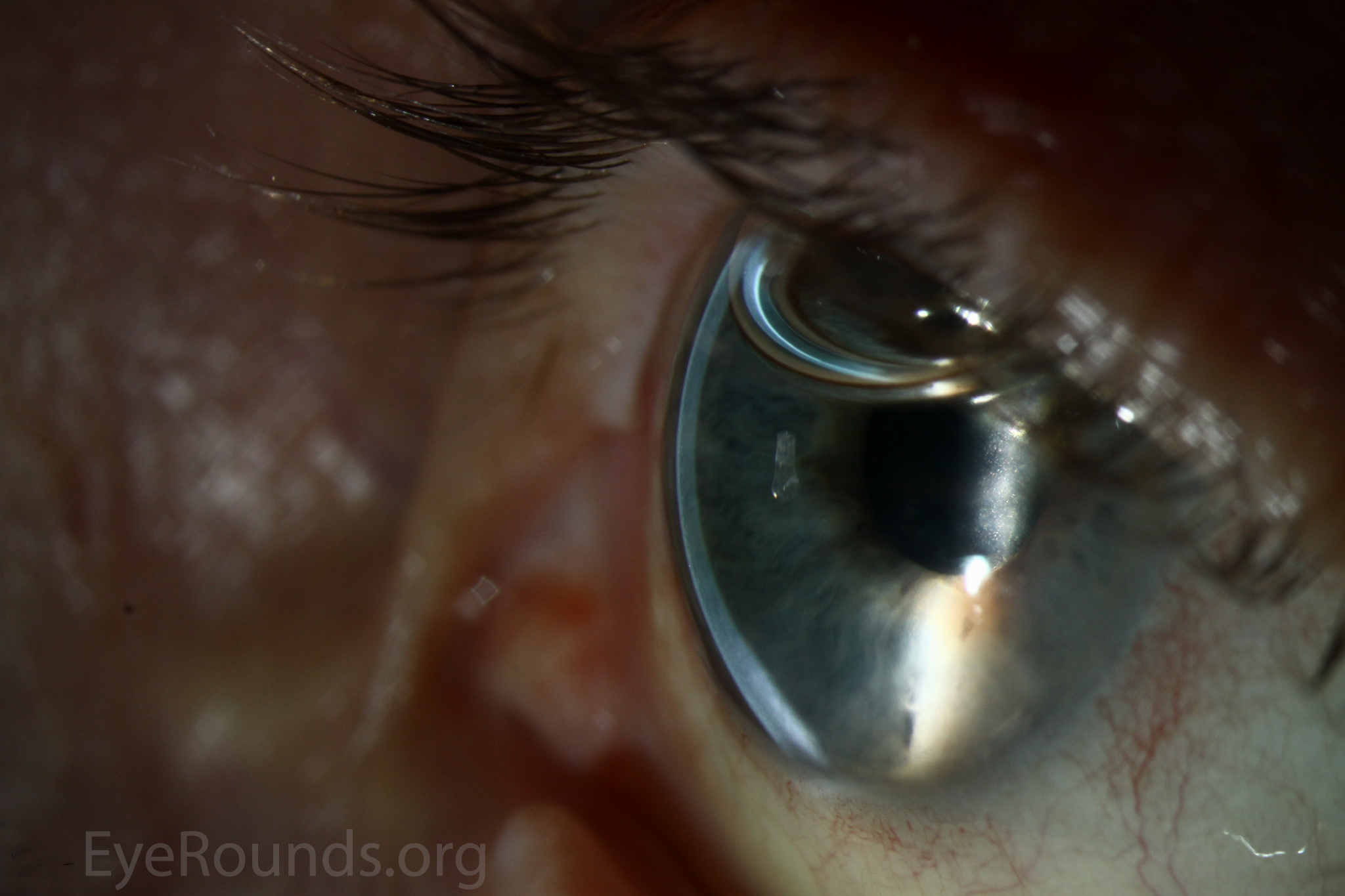
The indications for DMEK are similar to those for DSAEK, including endothelial dystrophies (such as Fuchs corneal dystrophy and posterior polymorphous corneal dystrophy), pseudophakic bullous keratopathy, ICE syndrome, and other causes of corneal endothelial dysfunction (1, 10, 17).
DMEK offers the most rapid visual rehabilitation of any keratoplasty technique to date (Figure 12). Final visual acuity can be outstanding due to minimal optical interface effects. Because less tissue is transplanted, there is a lower risk of allograft rejection and less long-term reliance on topical steroids compared with other types of keratoplasty. Discontinuation of topical steroids can be considered at or before 1 year after the procedure, especially for patients with elevated intraocular pressure.
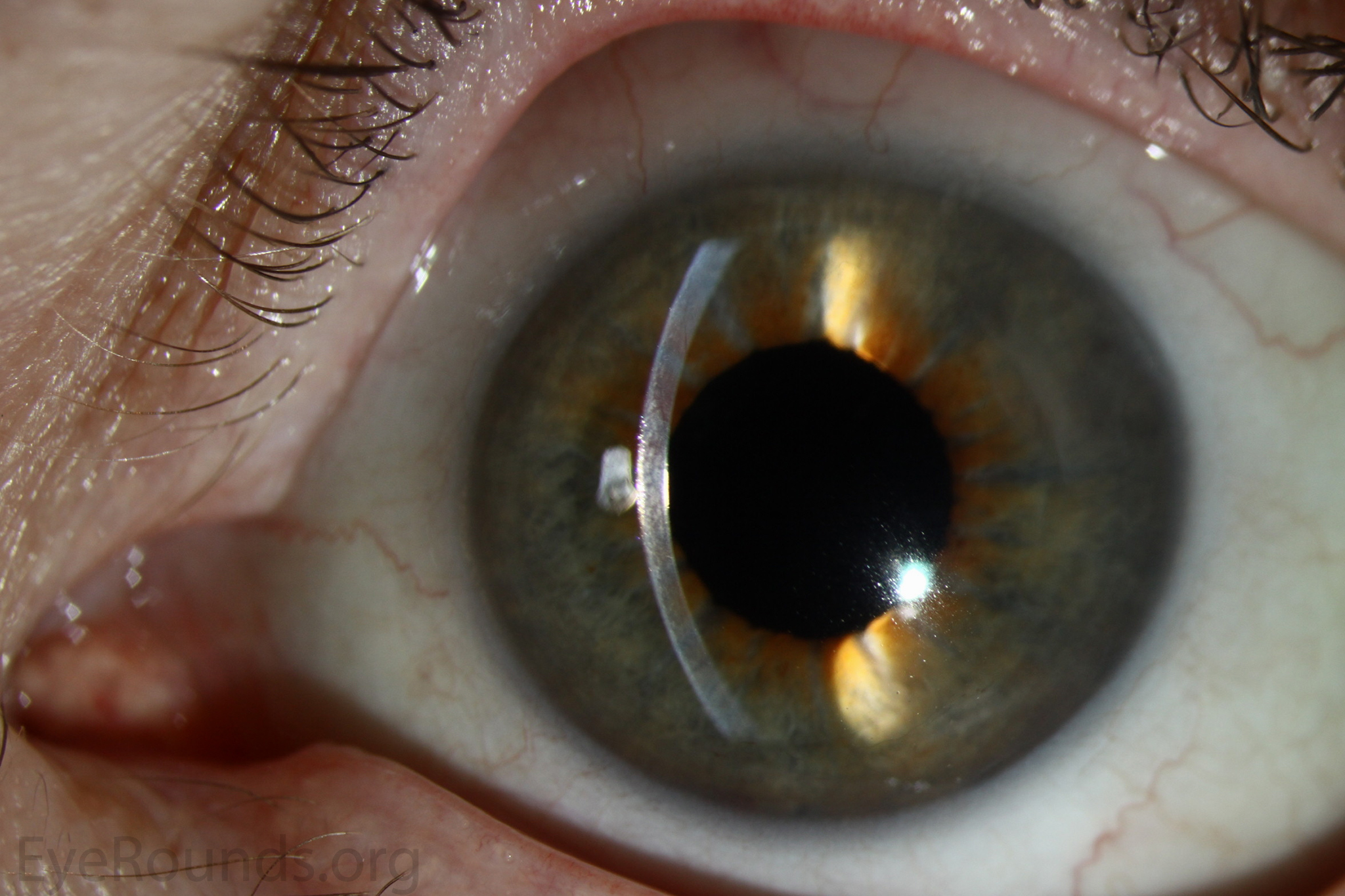
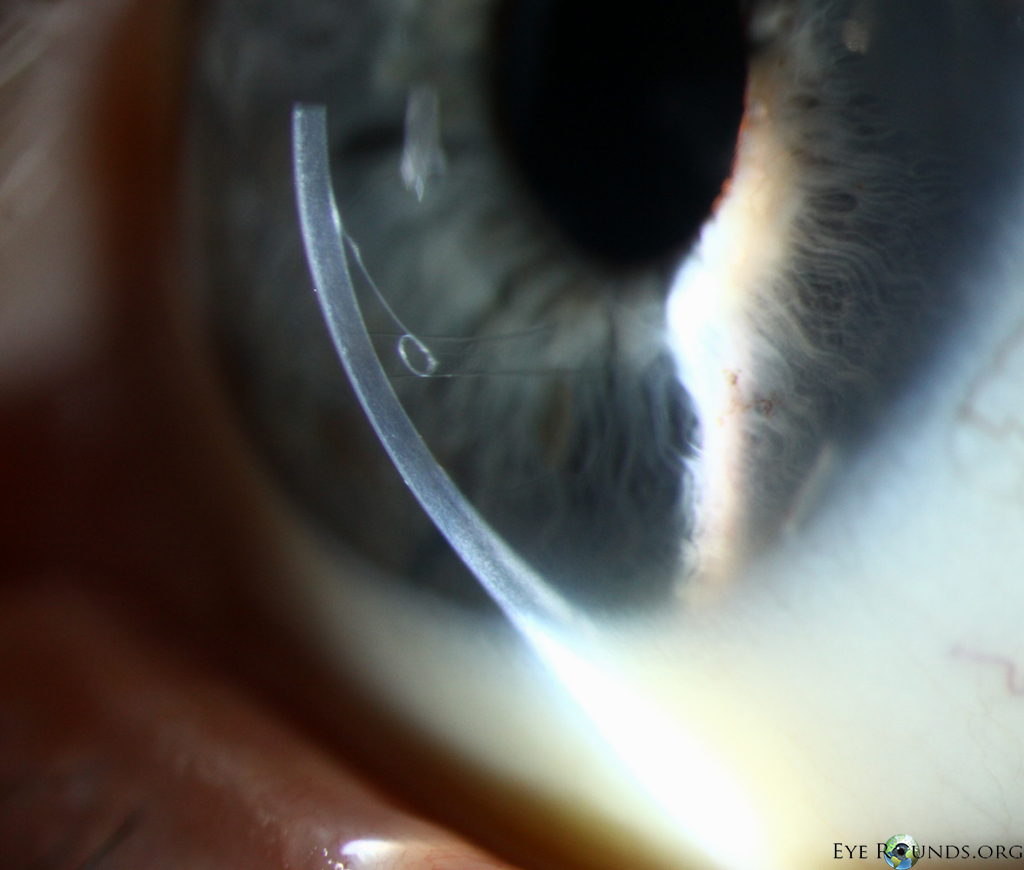
Because of thinness, fragility, and its characteristic scrolling properties (with the endothelium facing outward), the donor tissue can be difficult to handle and contribute to technical difficulties with the procedure. There is a higher risk of graft edge lifts (Figure 13) compared with DSAEK, sometimes requiring a re-bubble procedure.
Video 4: Phakic DMEK, If video fails to load or resize, use this link
Basic procedure steps (Video 4):
Additional Resources:
Video Links
Articles
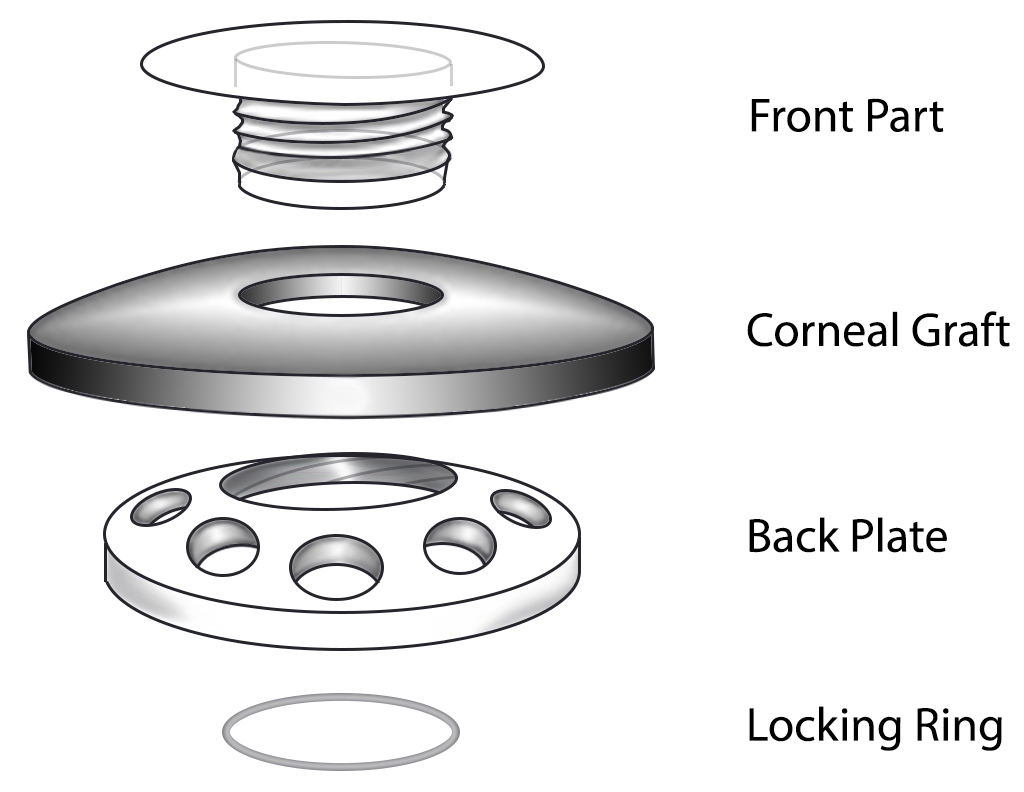
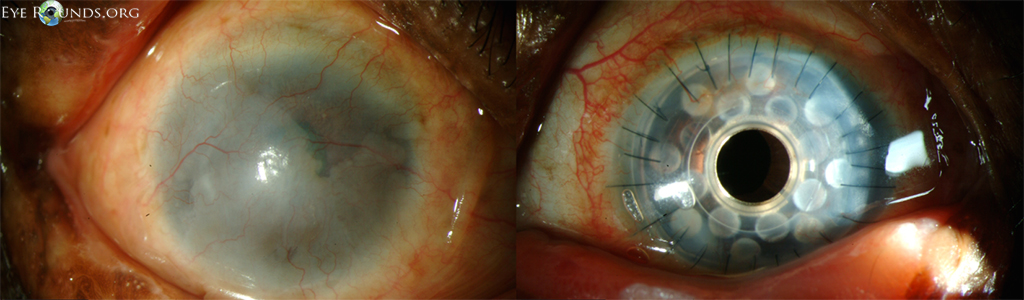
Keratoprosthesis implantation is a procedure that involves full-thickness removal of the cornea and replacement by an artificial cornea. The Boston Type I Keratoprosthesis is currently the most commonly used keratoprosthesis device in the US. It consists of a clear plastic polymethylmethacrylate (PMMA) optic and back plate sandwiched around a corneal graft and secured with a titanium locking ring (Figure 15). After the device is assembled, a partial-thickness trephination is performed on the host cornea. Full-thickness resection of the patient's cornea is then completed using curved corneal scissors. The keratoprosthesis is then secured to host tissue using interrupted or running sutures. Generally, patients who have a history of multiple failed PKs are candidates for a keratoprosthesis transplant. Other indications include severe keratitis or ocular surface disease resulting from limbal stem cell failure, such as Stevens-Johnson syndrome (Figure 16), ocular cicatricial pemphigoid, aniridia (Figure 17) and chemical injury (1, 13). The Boston Type II Keratoprosthesis is a similar device with a longer optic designed to extend through an opening made in the upper eyelid (Figure 19). It is indicated for the most severe cicatrizing ocular surface diseases.
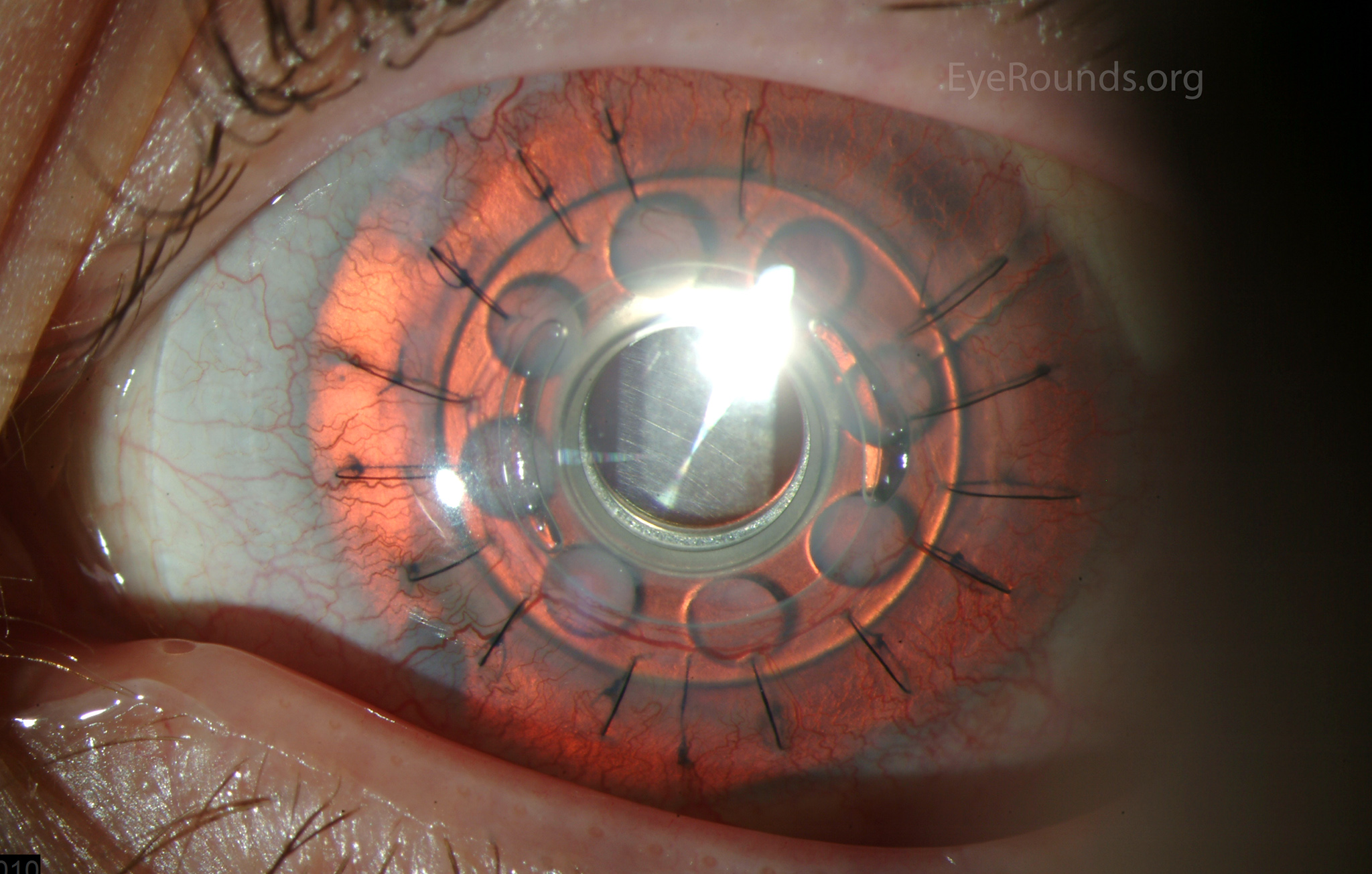
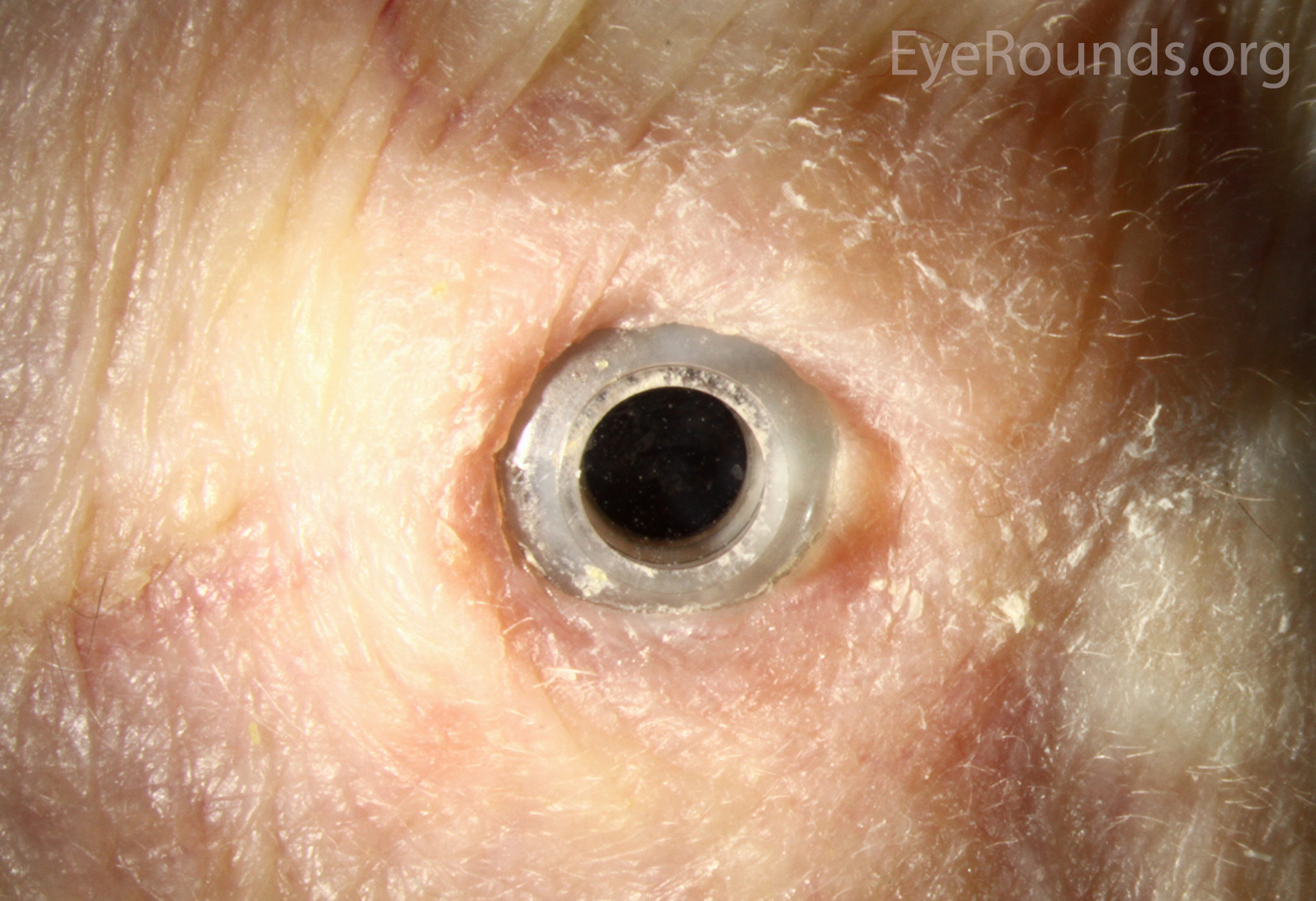
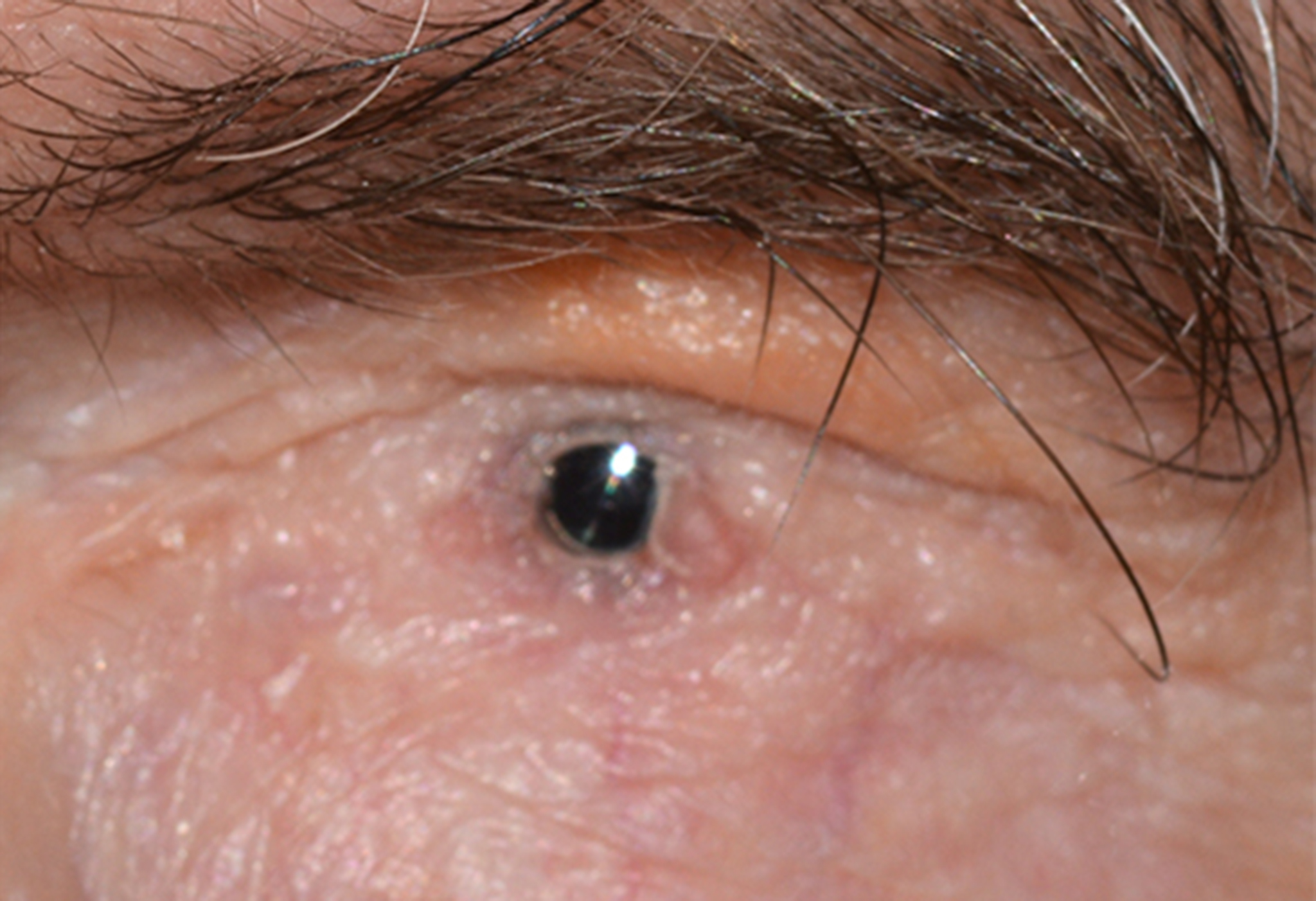
KPro placement offers relatively fast visual rehabilitation. The devices are amenable for use in many situations in which other types of keratoplasty are not an option.
There is significant long-term risk of complications for those with a keratoprosthesis. Because the KPro is a foreign body, there is risk of infection or extrusion of the device. Post-operative glaucoma is common and intraocular pressure is difficult to evaluate as the hard optic makes traditional tonometry impossible. For this reason, glaucoma tube shunts are typically placed at the time of the corneal transplant at the University of Iowa. The Diaton is currently the preferred way to measure intraocular pressure in these patients in our institution. Patients can form retroprosthetic membranes requiring treatment with a Nd:YAG laser or surgical membranectomy (21).
Basic procedure steps (Video 5):
Additional Resources:
Video Links
Articles
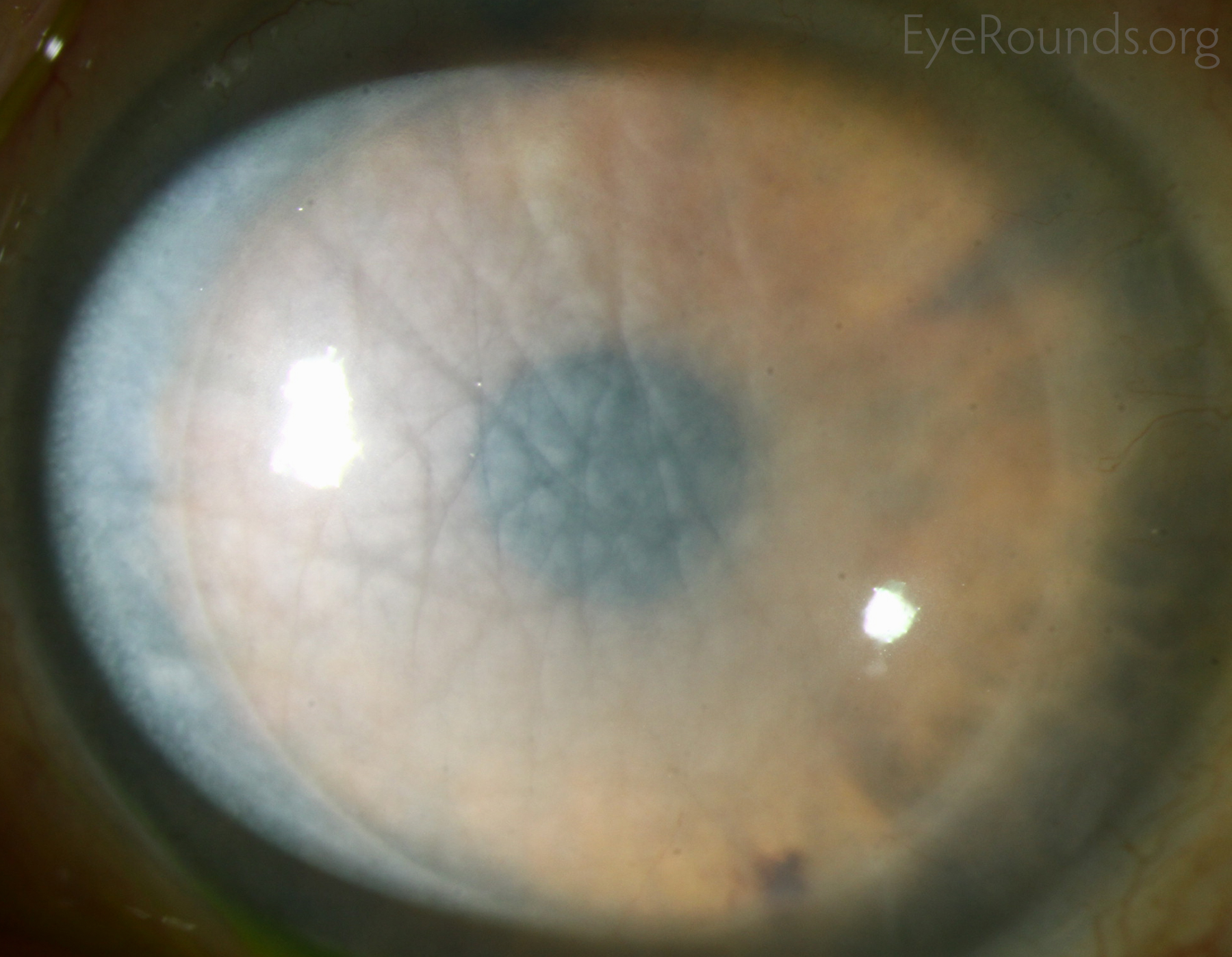
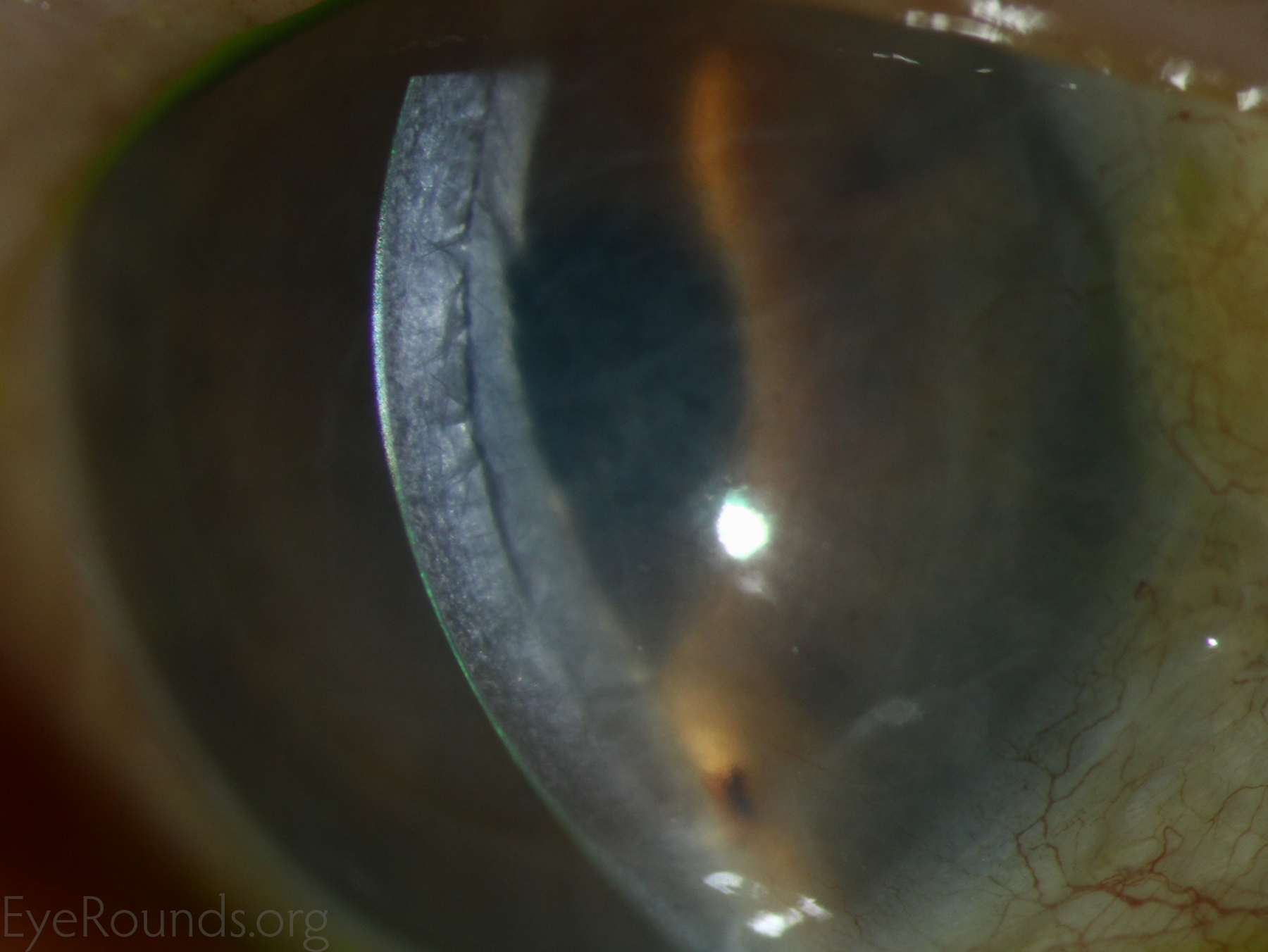
Corneal transplantation is regarded as the most successful solid organ transplantation procedure (1). Niziol et al. performed a study in 2013 with follow-up averaging 10 years and found that corneal rejection after PK for keratoconus occurred in 44% of grafts, but only 8% of grafts actually failed (22). While long-term rejection data is not yet available for the newer EK procedures, lesser rejection rates have been demonstrated after DMEK (0.7%) and DSAEK (9%) than PK (17%) at 2 years in patients on the same postoperative steroid regimen and treated for similar indications (23). This may be secondary to reduced antigen load in the thinner graft tissue. Modern treatment efforts can account for the vast difference between graft rejection and failure. However, graft rejection still remains a significant cause of corneal graft failure (Figure 16) (1). The most effective intervention is early recognition and prompt treatment with topical steroid drops. If the patient notices any redness, pain, or decreased vision, it is critical to seek prompt treatment to maximize chances of reversing the rejection episode.
Donaghy CL, Vislisel JM, Goins KM, Greiner MA. An Introduction to Corneal Transplantation. May 21, 2015; Available from: https://eyerounds.org/tutorials/cornea-transplant-intro/