 |
|
|
|
White Noise Field Campimetry
|
|
change in brightness perception (possible states are: darker, equal, brighter) | |
|
change in noise perception, i.e. in the “apparent” movement of dots (possible states are: increased, unchanged, reduced, deficient). |
The border of the “cloud” is indicated by the patient with the tip of his finger directly on the surface of the monitor and can be directly documented by a touch screen option (or – in former version – by directly following the path of the patient´s indicating finger with a computer mouse).
For clinical evaluation, white noise field campimetry was compared to results of threshold-related, slightly supraliminal automated static grid perimetry, obtained with the Tuebingen Automated Perimeter (TAP). With this conventional perimetric technique a comparatively high spatial resolution (191 test location with centripetal condensation and polar arrangement within the central 30° visual field) is achieved at the cost of an ultimate scotoma depth resolution.
Test times of white noise field campimetry range from 1 to 5 minutes per eye, including standardized questions about the perception of the noise field and documentation of the result.
Extensive studies have been performed in order to test whether white noise field campimetry is capable of detecting visual pathway lesions of various origin (see chapter “Sensitivity and Specificity”).
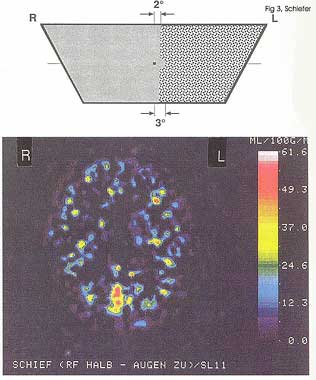
PET as well as fMRI studies (Fig. 3) were able to show that a (hemifield) random noise stimulus is conducted to the visual cortex and is able to elicit specific activity in the contralateral hemisphere 6.
The specificity of white noise field campimetry was estimated by presenting this stimulus to 198 eyes of 198 opthalmologically normal subjects (age range 11 to 74 years)7. Subjective statements, which characterized the flicker perception as “completely normal over the entire screen” (n = 84) or described a “somewhat changed” flicker perception at the very edges of the campimeter (n = 21) or a “more distinct” flicker perception in the central snowfield area (n = 19) or finally reported on “transient clouds”, which disappeared immediately after blinking (n = 19), were regarded as normal. This evaluation procedure resulted in a specificity of 82.3% (95-C.I. 76.9 – 87.1%).
Sensitivity for defect detection was investigated with subjects having white noise field campimetry and conventional automated static threshold-oriented, slightly supraliminal perimetry (30° TAP, see chapter “Methods”) on the same day in 368 eyes of 368 patients with visual pathway lesions from various origins. Overall scotoma detection rates were 80.7% (95%-C.I. 76.7% - 84.3%) for white noise field campimetry, compared to 84.2% (95%-C.I. 80.5% - 87.6%) with conventional TAP perimetry. In the vast majority, noise field defects were noticed by combined changes in noise (most frequently reduced or even defective) and brightness perception (with reports on “dark” or “bright” clouds being roughly equally distributed).
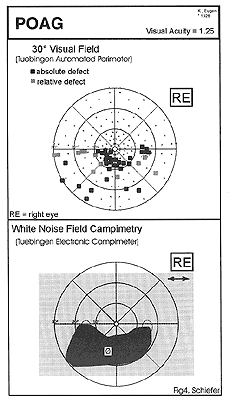
In contrast to conventional static perimetry, sensitivity of white noise field campimetry turned out to highly dependent on e.g. scotoma depth and also location (i.e. site) of the lesion. Relative glaucomatous visual defects in TAP perimetry (, i.e. stage I, according to the Aulhorn classification 8) were detected by white noise field campimetry in 65.0% of cases. With absolute glaucomatous scotomas, detection rate was 83.8% without connection to the blind spot (Aulhorn stage II) and increased to 100% for all stages of more advanced glaucomatous visual field loss. A typical finding is shown in Figure 4.
In 162 eyes with homonymous visual field defects due to the post-chiasmal visual pathways lesions, detection rate of conventional TAP perimetry was 98.8% (95%-C.I. 95.7% - 99.9%), whereas sensitivity of white noise field campimetry was only 76.5% (95%-C.I. 70.5% - 82.2%)1. More recent studies give evidence for higher detection rates of white noise field campimetry for those cases with involvement of the visual cortex 9.
To the author´s knowledge, no systematic studies addressing reliability and re-test variability of white noise field campimetry have been undertaken so far.
A “floor” effect, i.e. missing detection at the low end of the dynamic range, does exist insofar as very subtle relative defects may be not detected by the patients (see also chapter “Sensitivity and Specificity”). Furthermore, the method seems to have a reduced or even missing sensitivity towards specific conditions of visual pathway lesions (e.g.: extensive retinal lesions or long-standing post-geniculate lesions; this aspect is also addressed in “Sensitivity and Specificity” chapter).
A “ceiling” effect is found in case of subtotal visual field loss, most probably due to fixation problems and a remnant normal area, which may be too small for acting as a sufficient reference region. Subjects with inability to fixate (e.g. due to nystagmus) or profound central scotomas may be unable to perceive the noise field stimulus in an adequate manner.
Furthermore, even advanced visual field loss located outside the video display cannot be detected.
The test is practical, requiring a black and white TV monitor without a broadcast reception; in the high end version, a calibrated, high-resolution colour monitor with integrated touch screen, a control monitor, and a video-pupillographic device have to be integrated in an appropriate computer system graphics card is used. A custom built lens holder that fits on the top of the monitor along with custom made lenses is necessary.
To the author's knowledge, no systematic studies addressing subject acceptance have been published so far. Our experience is that most of the patients had no difficulty with the test.
White noise field campimetry is a subjective method in twofold ways: on the one hand it depends on the intellectual abilities of the examined subject to perceive and adequately describe the observed phenomena, on the other it is influenced by the recognition and interpretation of the examiner. Further errors can be introduced by the fact that patients, who for first time in their life perceive their scotomas, will tend to directly look at these phenomena. This spontaneous gaze movement inevitably shifts the scotoma, which might then be dislocated outside the examination area and thus disappear. Careful, fixation continuous control by the examiner is therefore mandatory in this method.
Subjects with improper correction, inability to fixate or profound central scotomas may not be able to perceive the noise field stimulus in an adequate manner.
As with conventional automated perimetry, tear film, eye brow and eye lash artifact can occur.
Specificity data is available on 198 eyes of 198 ophthalmologically normal subjects (age range 11 to 74 years)7 1. Sensitivity data were obtained from 368 eyes of 368 patients with various pathway lesions (see chapter “Sensitivity and Specificity”)7.
In a recent version the noise field stimulus is exclusively software generated and presented on a calibrated, high-resolution colour monitor with integrated touch screen; the results are recorded on a separate examination screen 1;5 (Fig. 2). This enables the patients to delineate their scotomas on the examination monitor without any disturbance by visible borders. Noise field characteristics (i.e. brightness, movement of the dots and colour characterstics) within the demarcated scotoma region(s) or within the entire examination screen can be interactively varied in order to assess further quantitative scotoma features.
In a pilot study white noise field stimulus has also been used for remote screening via a regular TV program 10;11.
Originally developed by: Elfriede Aulhorn and Gert Köst
Further developments by: Ulrich Schiefer and Guido Stercken-Sorrenti
Commercially available: Please contact OCULUS Inc., Dutenhofen, Germany or further information; for screening purposes, a regular black and white TV screen with no broadcast reception seems to be sufficient.
 |
| Figure 1: Original set-up of white noise field campimetry using a black and white TV screen without broadcast reception. (back to text) |
|
|
| Figure 2: Current version of the Tuebingen Computer Campimeter (TCC), which is capable of presenting a software-generated noise-field stimulus on a calibrated, high-resolution, colour monitor with integrated touchscreen (right). The fixation of the patient is recorded by an infrared video camera and shown on a separate monitor. A third monitor is used for visualization of the examination result, which can be stored and printed. (back to text) |
 |
|
Figure 3: Top: (Left) hemifield presentation of white noise field stimulus (sparing the visual field center); in the right hemifield (including the paramedian region) a homogenous isoluminant area is presented Bottom: Corresponsing fMRI result showing a clear activation of the occipital visual cortex, contralateral to the snowfield hemifield. (back to text) |
 |
|
Figure 4: Typical finding in glaucomatous visual field loss. Top: Conventional, automated threshold-related slightly supraliminal static perimetry (30° Tuebigen Automated Perimeter), showing an inferior arcuate nerve fiber bunde defect Bottom: Corresponding result of white noise field campimetry. According to the description of the patient the noise field defect is described as a “cloud”, which is darker than the surrounding normal snowfield and characterised by a reduced apparent movement of the dots within this area. (back to text) |
1. Schiefer U. Rauschfeldkampimetrie. Stuttgart: Kohlhammer, 1995.
2. Aulhorn E. White noise field campimetry. Lecture at 85. German Ophthalmological Society Meeting. 1987.
3. Aulhorn E, Köst G. Rauschfeldkampimetrie. Eine neuartige perimetrische Untersuchungsmethode. Klin Monatsbl Augenheilkd 1988;192:284-8.
4. Aulhorn E, Köst G. In: Heijl A, ed. Perimetry Update 1988/89. Proceedings of the VIIIth International Perimetric Society Meeting. Amsterdam: Kugler & Ghedini, 1989: 331-6.
5. Schiefer U, Stercken-Sorrenti G. Ein neues Rauschfeldkampimeter. Klin Monatsbl Augenheilkd 1993;202:60-3.
6. Schiefer U, Skalej M, Kolb R et al. Cerebral activity during visual stimulation - a positron emission tomography and functional magnetic resonance imaging study. German J Ophthalmol 1996;5:109-17.
7. Schiefer U, Pfau U, Selbmann HK et al. Sensitivität und Spezifität der Rauschfeldkampimetrie. Ophthalmologe 1995;92:156-67.
8. Aulhorn E, Karmeyer H. Frequency distribution in early glaucomatous visual field defects. Docum Ophthal Proc Series 1977;14:17-83.
9. Kolb M, Petersen D, Schiefer U et al. Scotoma perception in white-noise-field campimetry and postchiasmal visual pathway lesions. German J Ophthalmol 1995;4:228-33.
10. Gisolf AC, Kirsch J, Selbmann HK, Zrenner E, Schiefer U. in: Wall M, Heijl A, eds. Perimetry Update 1996/1997. Amsterdam: Kugler Publications, 1997: 49-50.
11. Schiefer U, Gisolf AC, Kirsch J et al. Rauschfeld-Screening - Ergebnisse einer Fernseh-feldstudie zur Detektion von Gesichtsfelddefekten. Ophthalmologe 1996;93:604-16.
![]()
![]()
Copyright 2008. Imaging and Perimetry Society