Current Concepts in the Diagnosis and Management of Meibomian Gland Dysfunction-Related Evaporative Dry Eye Syndrome
Tyler S. Quist, MD and Michael D. Wagoner, MD, PhD
Posted March 13, 2018
Chief Complaint
Worsening burning and irritation of both eyes after several months of "dry eye" treatment
History of Present Illness
This patient is a 62-year-old Caucasian woman with a diagnosis of aqueous tear deficiency who had no improvement in her dry eye symptoms despite several months of aggressive lubrication, bilateral lower punctal plugs, and the use of topical cyclosporine 0.05% (Restasis®).
She had a long-standing history of chronic burning and foreign body sensation, photophobia, mild morning discharge, intermittent blurry vision, asthenopia, and bilateral monocular diplopia. Her symptoms were exacerbated by blowing air and prolonged visual tasks and were modestly improved with the intermittent use of hot compresses, the removal of fans from the immediate environment, and the introduction of home humidifiers. Topical lubricants provided transient relief of the foreign body sensation but had no efficacy for her other symptoms. Her Standardized Patient Evaluation of Eye Dryness (SPEED) score was 18.
Past Ocular History
- No ocular surface disorders, intraocular inflammation, or contact lens use
- No ophthalmic surgeries
Past Medical History
- Obstructive sleep apnea on continuous positive airway pressure (CPAP) therapy
- Hypothyroidism
- Non-Hodgkin's lymphoma
- No autoimmune disorders (tested negative for Sjögren's syndrome), atopy, or collagen-vascular disease
Medications
- Cyclosporine 0.05% (Restasis®), both eyes, twice a day
- Topical lubricating drops every hour to 2 hours while awake
- Bedtime lubricating ointment as needed
Family History
- No Irish ancestry
- No atopy
Social History
- Non-contributory
OCULAR EXAMINATION
Best-corrected visual acuity (BCVA)
- Right Eye (OD): 20/25. Of note, visual acuity intermittently fluctuated to 20/40 OD during visual acuity testing without changes in manifest refraction.
- Left Eye (OD): 20/25. Of note, visual acuity intermittently fluctuated to 20/40 OD during visual acuity testing without changes in manifest refraction.
Pupils, intraocular pressure, motility, and confrontational visual fields
- Normal in both eyes (OU)
Eyelids
- Incomplete blink OU
- Good lower eyelid position without laxity OU
- Mild debris on eyelids OU
- Meibomian gland expression (MGE) score: 2 OD; 2 OS
- Mild oil inspissation (toothpaste-like secretions) OU
- Lower plugs in place OU
Conjunctiva
- 1+ diffuse injection OU
- Mild papillary reaction OU
- Mild conjunctivochalasis OU
Cornea and pre-corneal tear film
- Tear meniscus: normal height without mucus or debris OU
- Rapid tear film break up time (TFBUT): 4 seconds OD; 3 seconds OS
- Fluorescein stain score (FSS): 3 OD; 3 OS
- Inferior cornea: 3+ OD; 3+ OS
- Interpalpebral fissure: zero OU
- Corneal stroma: clear and compact without marginal infiltrates OU
- Anterior chamber: deep and quiet OU
- Lens: trace nuclear sclerosis OU
- Fundus: normal OU
Ancillary Studies
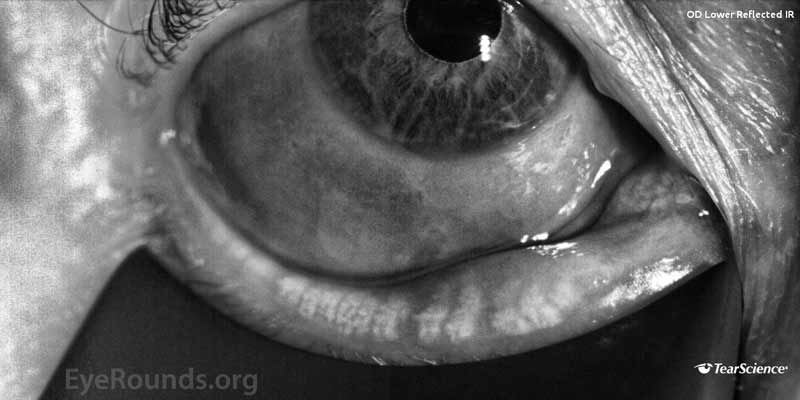
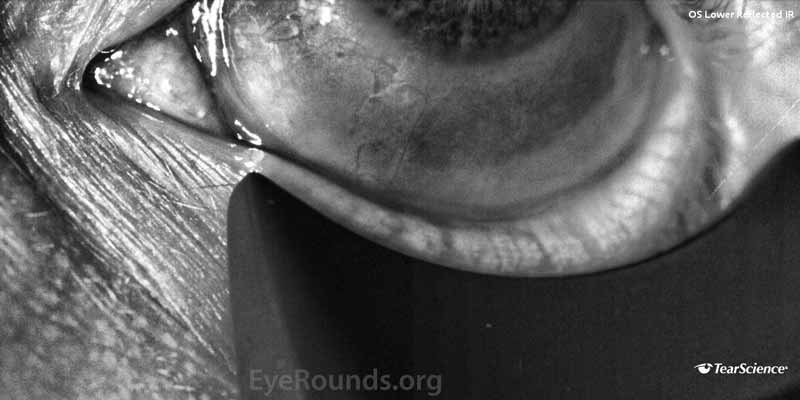
- LipiView® (Tear Science, Morrisville, NC, USA) blink analysis demonstrated a 100% incomplete blink OU with continuous exposure of the inferior 1/3 of both corneas as shown in Figure 1.
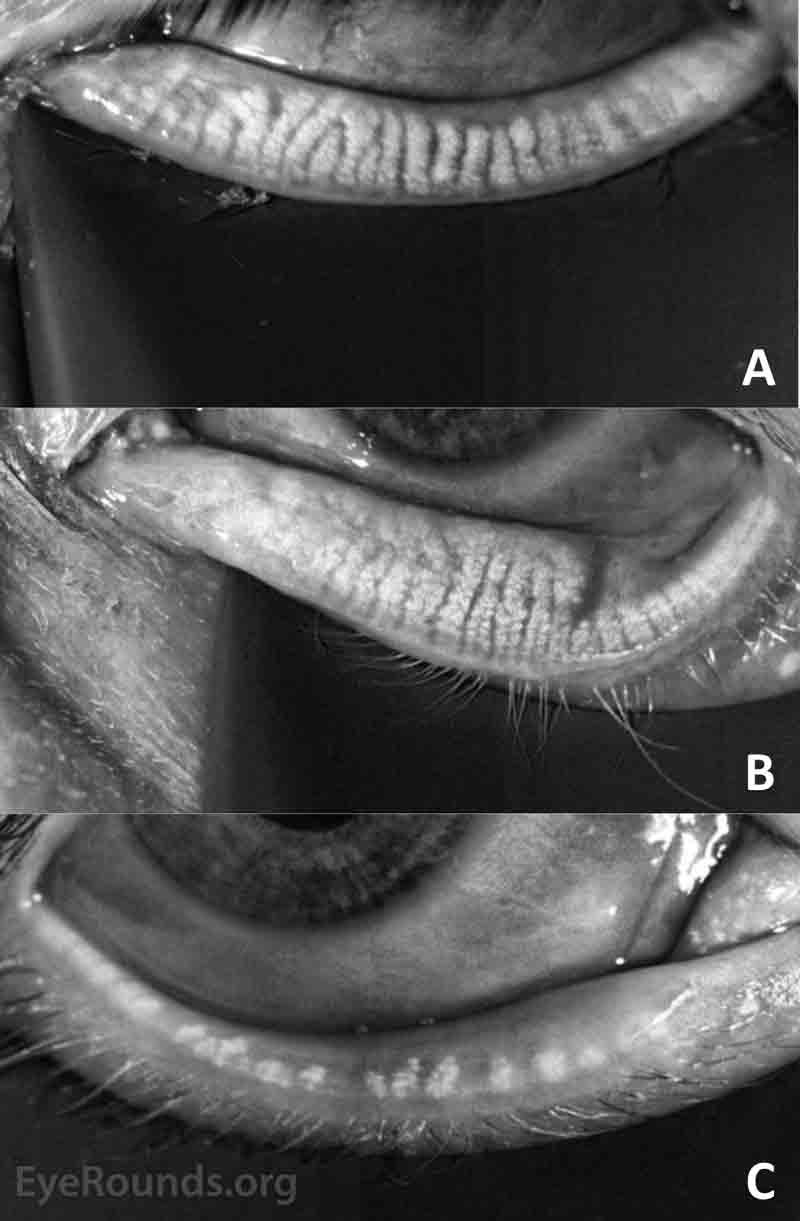
- LipiView® meibography demonstrated mild dilation and gland tortuosity with some loss of terminal gland architecture in the right lower eyelid and moderate gland dilation and tortuosity was present in the left lower eyelid with extensive loss of terminal gland architecture, including complete loss of two glands as shown in Figure 2.
Figure 1A
If video fails to load, use this link: https://vimeo.com/258641168
Figure 1B
If video fails to load, use this link: https://vimeo.com/258641929
Figure 1. (A) Blink analysis of the right eye and (B) blink analysis of the left eye.
Figure 2. (A) Meibography of the right lower eyelid characterized as mild disease and (B) meibography of the left lower eyelid characterized as moderate disease.
DIAGNOSIS
Combined mechanism dry eye syndrome
- Meibomian gland dysfunction (MGD)-related evaporative dry eye syndrome: predominant component
- Aqueous tear deficiency (ATD)-related dry eye syndrome: minor component
Initial Therapy
- Manual meibomian gland expression in the clinic
- For the MGD-related evaporative dry eye component, the patient began:
- Hot compresses and massage OU once or twice a day
- Daily lid hygiene
- blink exercises OU 6 to 10 times a day
- Fish oil supplements 1000 mg, daily
- Fluorometholone 0.1% (FML®) OU twice a day
- Erythromycin ointment OU at bedtime
- Control environmental modification
- For the ATD-related dry eye component, the patient continued:
- Restasis® OU twice a day
- Lower eyelid punctal plugs OU
- Topical lubricants OU as needed
CLINICAL COURSE
There was mild symptomatic improvement with a reduction of the SPEED score to 12 after 4 months of strict compliance with the therapeutic regimen. However, the subjective visual complaints and foreign body sensation did not improve. The BCVA remained 20/25 in both eyes with episodic reduction to 20/40. The MGE and FSS scores and TFBUT were unchanged. One 12 minutes treatment was provided with the LipiFlow® Thermal Pulsation System (Tear Science, Morrisville, NC, USA). The outpatient regimen was not changed.
Two months later, all subjective symptoms had resolved. The SPEED score was zero. The BCVA was 20/15 with no fluctuation. The MGD and FSS scores were both zero. The TFBUT was > 7 seconds in both eyes. Daily fish oil supplements, hot compresses and digital massage, lid hygiene, and blink exercises were continued. Topical FML® and erythromycin were discontinued.
DISCUSSION
The current case provides a classic example of a patient with "dry eyes" who has failed to respond to therapy. Failure to respond to dry eye therapy often results from the absence of therapy, either from patient non-compliance or physician failure to properly diagnose the etiology and provide appropriate interventions.[1-4] The clinical course highlights the difficulty encountered in reestablishing meibomian gland patency with conventional therapy and the additional benefit provided with adjunctive gland opening maneuvers, such as LipiFlow® therapy, especially in eyes with reasonable gland preservation.[5-12]
Etiology and Epidemiology
Dry eye syndromes occur when abnormalities of the pre-corneal tear film result in inadequate lubrication of the ocular surface.[1-4] Evaporative dry eye, either alone or in combination with insufficient aqueous tear production, accounts for up to 85% of all cases.[1] The cause of evaporative dry eye is MGD in which there is a quantitative oil deficiency due to obstruction-related hyposecretion and a qualitative oil deficiency due to production and secretion of poor quality oil. These abnormalities lead to signs and symptoms that can be directly attributed to tear film instability. There may or may not be additional signs and symptoms due to MGD-associated ocular surface inflammation and bacterial overgrowth.
Primary MGD occurs as part of normal aging and is prevalent in older populations.[1-4] The condition is usually asymptomatic, but up to 10-15% of patients over the age of 50 years have symptomatic evaporative dry eye.[1] The prevalence of symptomatic disease appears to be higher in men and is more common in Asian populations compared with Caucasian populations.[13,14]
Secondary MGD is especially common in patients with acne rosacea, chronic atopy, mechanical or neurologic abnormalities of the eyelids, or any condition associated with acute and severe ocular surface inflammation (e.g., chemical injury) or chronic ocular surface inflammation of any severity (e.g., prolonged ATD, graft-versus-host disease, cicatricial conjunctival disorders).[1-4]
Pathophysiology
Meibomian glands are sebaceous glands within the tarsal plates of the eyelids. There are approximately 30 and 25 glands in the upper and lower eyelids, respectively.[15] These glands are composed of clusters of acini, and they measure 5.5 and 2 mm in length in the upper and lower eyelids, respectively.[15] Contraction of the orbicularis oculi muscle facilitates glandular secretions that make up the outer lipid layer of the pre-corneal tear layer and are composed of lipids, proteins, and electrolytes.[16]
Most commonly, MGD is caused by obstruction-related hyposecretion.[17] The underlying mechanism involves epithelial hyper-keratinization that causes duct obstruction, stasis, cystic dilation, and, ultimately, glandular atrophy.[17] Numerous factors, including aging, rosacea, nutritional status, alterations in sex hormones, blink abnormalities, and inflammation, contribute to qualitative deficiencies in the secretion of meibomian gland oils.[17] Rosacea is a dermatologic disease associated with overexpression of cathelicidins that contribute to neutrophil recruitment and cytokine release causing glandular dysfunction of the face and eyelids. Nutritional disorders such as malnutrition, protein deficiency, vitamin A deficiency, and a diet low in omega-3 fatty acids have all been associated with poor tear film health.[18] Androgens deficiency has been associated with MGD while the effect of estrogen and progesterone on dry eye remains controversial.[19]
Quantitative and qualitative deficiencies of meibomian gland secretions are the main causes of evaporative dry eye disorder. They contribute to instability of the outer lipid layer of the tear film, increased tear evaporation, and tear hyperosmolarity. In addition, qualitative deficiencies of the glands contribute to ocular surface inflammation and bacterial overgrowth on the eyelid margins.
Signs and Symptoms
The predominant symptoms of MGD-related evaporative dry eye syndrome are attributable to tear film instability.[1-4] The most common symptom is visual fluctuation. This is most noticeable during visual tasks that are associated with decreased blinking, such as reading, using the computer, watching television, and driving. Subjective complaints are related to variable induction of irregular astigmatism and include visual fatigue, blurred vision, reduced focusing ability, and bilateral, monocular diplopia. Despite the presence of dry eye, paradoxical reflex tearing may occur with normal lacrimal gland function, particularly in patients experiencing evaporative conditions, which include exposure to low environmental humidity and blowing air. Some patients complain of foreign body sensation if corneal epithelial desiccation occurs as a result of excessive evaporation not sufficiently compensated for by reflex tear production. The symptoms of tear layer instability can only be temporarily relieved by blinking or the administration of ocular lubricants. The SPEED survey is an effective method for assessing the severity of these symptoms on a scale of zero (asymptomatic) to 28 (maximum symptoms) as shown in Figure 3.[20,21]
Figure 3: SPEED survey
The signs that correspond to the symptoms suggestive of tear film instability include reduced visual acuity, evidence of meibomian gland inspissation, rapid TFBUT, and punctate epithelial erosions (PEE). Highly variable subjective responses are often noted during refraction. As with the evaluation of the cataract patient, the range of visual acuities should be documented. Although the best-corrected acuity is often normal in mild cases, it may be decreased in moderate-to-severe cases owing to tear film-related irregular astigmatism and/or punctate epitheliopathy in the visual axis.
Meibomian gland expressibility (MGE) is evaluated by determination of the number of glands that can be expressed with mild pressure with a cotton-tipped swab or a commercially available device that is specifically formulated for this purpose. Five glands in the lateral, middle, and nasal thirds of the lower eyelid (total for each eyelid is 15 glands) are expressed and scored.[22] Examples are shown in Table 1. A score of zero indicates a complete absence of glandular expression and a score of 15 indicates that all glands are expressible throughout the lower eyelid. Patients with MGE scores of zero to two are almost invariably symptomatic, and those with a score of 7 or greater are usually asymptomatic. An evaluation of meibomian gland secretions is performed (and a therapeutic intervention is simultaneously provided) with vigorous expression of occluded glands of both the lower and upper eyelids with firm pressure with a cotton-tipped swab. The secretions that are obtained are graded along a continuum from clear to "cheese- or toothpaste-like," with a linear correlation between progressive loss of fluidity and symptomatic disease.
| Number of glands expressed in the nasal third of the eyelid (0-5) | Number of glands expressed in the middle third of the eyelid (0-5) | Number of glands expressed in the lateral third of the eyelid (0-5) | Meibomian gland score (0-15) |
| 0 | 0 | 0 | 0 |
| 2 | 3 | 1 | 6 |
| 5 | 5 | 5 | 15 |
Table 1. Examples of meibomian gland expressibility (MGE) scores.
Clinical evidence of the chronicity of the condition can be drawn by identifying keratinization and vascularization of the eyelid margin, loss of lashes, and evidence of a previous hordeolum or chalazion. Confirmatory evidence of both chronicity and permanent functional changes to the meibomian glands can be obtained with LipiView® meibography as shown in Figure 4. Normal meibomian anatomy is characterized by long vertical glands that extend from the eyelid margin to the end of the tarsal plate. The glands become dilated and tortuous near, especially near the upper tarsal margin in early and mild disease. In intermediate duration and moderate disease, the gland dropout progresses with loss of identifiable gland architecture and some complete loss of gland structures. In prolonged and severe disease, all glands are severely shortened and most may be missing. In each category, there is usually a gradient that extends across the eyelid from the punctum (most severe) to the lateral canthus (least severe).
Figure 4. (A) Mild, (B) moderate, and (C) severe anatomic changes of the meibomian glands.
The pre-corneal TFBUT is assessed by applying a fluorescein strip to the conjunctiva and using a slit lamp with cobalt blue illumination to measure the time between the last blink and the first dry cornea spot within the visual axis.[23] TFBUT times less than two seconds are almost invariably symptomatic, and those of >5 seconds are usually asymptomatic. The LipiView® imaging system can objectively measure the thickness of the lipid portion of the pre-corneal tear film. It uses the optical interference of light reflected off the tear film to determine the mean, maximum, and minimum thickness values (in microns) of the lipid layer over 20 seconds.[24,25] Although some studies have shown an inverse correlation between mean thickness and symptomatic disease, our own anecdotal experience has not supported these data.[24] Blink dynamics are evaluated by determining the frequency of partial and complete blinks, as well as imaging of the area of exposure with each complete blink during the testing interval.
PEE represent areas of desquamated surface epithelium that stain positively with fluorescein. The fluorescein staining score (FSS) is tabulated on a scale from zero to eight, with separate evaluation of PEEs in the inferior third of the cornea and the interpalpebral fissure on a scale of zero to four. A score that is greater than zero is usually symptomatic, with a linear correlation between the total score and the severity of ocular surface discomfort.
MGD-related evaporative dry eye syndrome may also have signs and symptoms related to ocular surface inflammation, including itching, burning, and photophobia. These symptoms tend be most severe in the morning because of poor clearance of the tear film while sleeping. They are usually worsened with the insertion of punctal plugs due to poor tear clearance. Itching may be present in any case, but it is more likely to occur and to be more severe in atopic patients. The most troublesome symptom is chronic burning with or without associated photophobia. This is presumably attributable to the presence of inflammatory mediators or to increased tear osmolarity in the pre-corneal tear film. The clinician is often faced with the paradoxical gap (i.e., the high symptom to sign ratio) between patient complaints, which may be dramatic, and objective signs of corneal epitheliopathy, which may be completely absent.
The most common acute sign of MGD-related ocular surface inflammation is marginal (peripheral) keratitis.[26] This is a hypersensitivity response to staphylococcal antigens that does not correlate with the severity of eyelid changes, especially in children in whom it may occur without any anatomic abnormalities. Chronic signs include conjunctival injection, conjunctivochalasis (especially in atopic patients), conjunctival foreshortening and symblepharon (especially in eyes with rosacea or cicatricial conjunctival disorders), and inferior PEE.
MGD-related evaporative dry syndrome may also be associated with bacterial overgrowth. Historically, this condition was once called "anterior blepharitis," as opposed to "posterior blepharitis," which was reserved for anatomic abnormalities of the meibomian glands. The symptoms include recurrent morning discharge with or without a history of recurrent conjunctivitis or hordeolum. On examination, there is often scaling, crusting, and debris on the eyelashes.Management
Meibomian gland dysfunction is a chronic condition that cannot be completely cured but can be treated. Previously published literature has reviewed current dry eye therapies to develop evidence-based management algorithms according to disease severity.[27] The only treatments that directly address the cause of this abnormality are those that relieve MGD and address tear film instability by improving the quantity and quality of the pre-corneal tear film. Treatment of associated conditions is often effective in relieving symptoms related to inflammation and bacterial overgrowth.
Patient education is an essential component of successful MGD therapy. In our Iowa City Veterans Affairs Medical Center (VAMC) practice, we provide our patients with verbal counseling regarding the pathophysiology and therapeutic principles of their disorder. This includes showing them their meibomian gland and precorneal tear film images, as well as the blink analysis. Detailed handouts, in lay language, on evaporative dry eye and ATD (if present) are provided and sent home to reinforce the information as shown in figure 4.
Figure 5. Dry eye patient handout.
Hot compresses and digital massage, once or twice daily, are the cornerstone treatment in the management of MGD.[6,7] Hot (at least 110°F/43°C) compresses, which are reheated as often as necessary, should be applied to the upper and lower eyelids for two to five minutes. While any regular washcloth may be used, Thermalon Dry Eye Moist Heat Compress is a popular option http://thermalon.com. Massage of the upper and lower eyelids should be done in conjunction with or immediately after the application of hot compresses. Even with strict patient compliance and good technique, there is often a suboptimal therapeutic in long-standing cases or in eyelids with markedly viscous oils. In some cases, improvement can be obtained with the use of commercially available eyelid warming devices, such as the MeiboPatch® (VISUfarma, Amsterdam, The Netherlands), and intermittent aggressive manual expression in the office of the eye care practitioner.
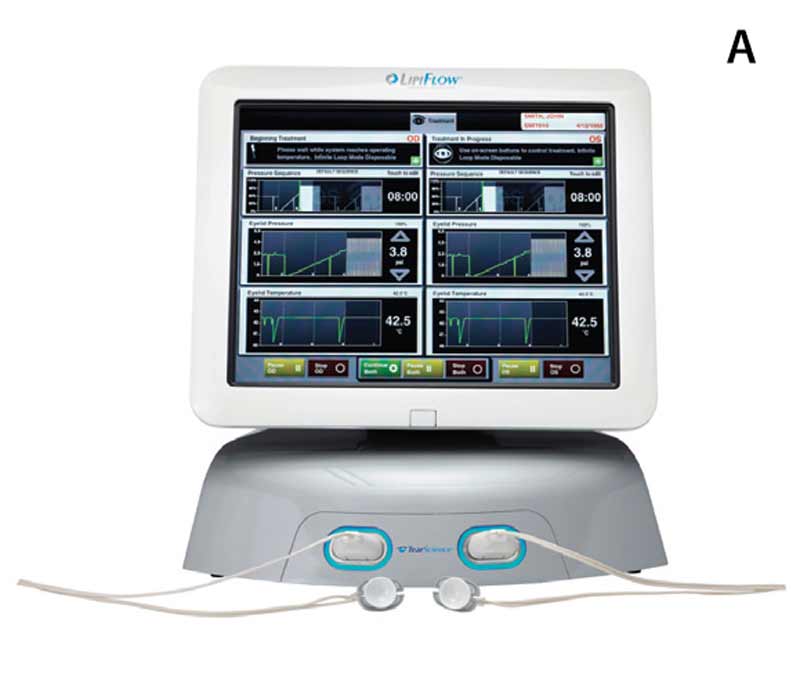
The recent introduction of LipiFlow® therapy provides, for the first time, a controlled Food and Drug Administration-approved method to further alleviate obstructed meibomian glands and to reduce the morbidity associated with evaporative dry eye syndrome.[8-13] The LipiFlow® machine, LipiView® screen, and eyelid warming devices are shown in Figure 6. The eyelid device has two parts: a lid warmer and an eyecup. The lid warmer is placed over the eye and heats the inside surface of the eyelids. The eyecup contains an inflatable device that massages the eyelids simultaneously. The procedure is 12 minutes and is reported by most patients to be pleasant and comfortable. Some patients experience a transient increase in ocular surface redness and irritation for a few days after the procedure.[5] Corneal abrasions, caused by mechanical trauma from the insertion of the device, have rarely been reported.[5]
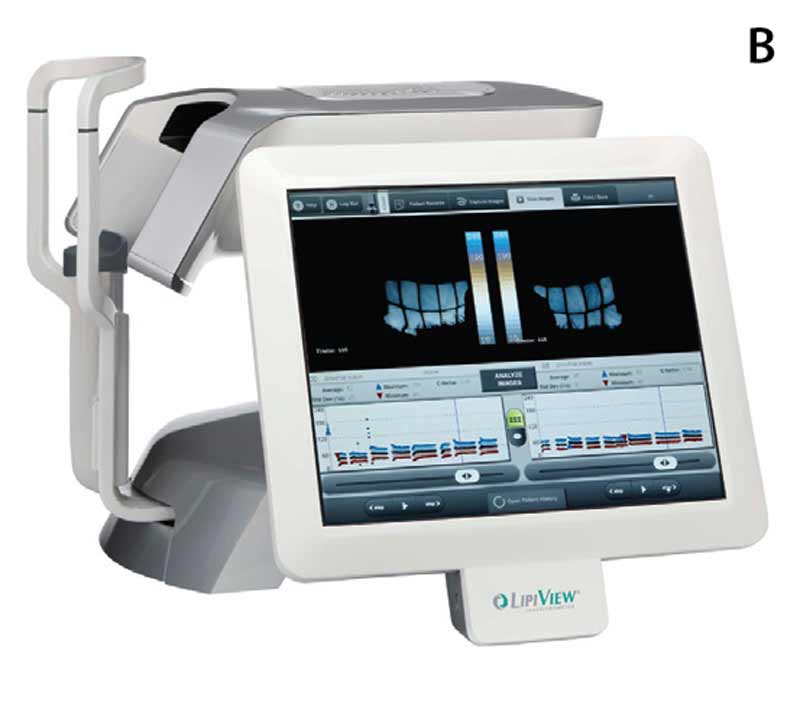
Figure 6. (A) LipiFlow® machine, (B) LipiView® screen, and (C) eyelid warming device on example patient.
Data from previously published studies suggest that a single LipiFlow® treatment may be associated with statistically better outcomes on multiple objective and subjective parameters, with effects lasting up to 36 months.[8-11] The efficacy of LipiFlow® therapy is directly correlated with an improvement in the MGE score.[8-11] A direct correlation exists between the degree of preservation of meibomian gland anatomy and subjective SPEED score improvements when evaluating the 125+ cases we have performed at the Iowa City VAMC, to date. However, there are considerable costs associated with the acquisition of the basic equipment, as well as the disposable applicators needed for each procedure. Because comparable MGE score improvements can sometimes be achieved with less expensive interventions, it is our practice to perform LipiFlow® therapy after documentation of failure of traditional strategies to alleviate gland obstruction.[12] For patients who elect to undergo LipiFlow® treatment, pre-treatment and post-treatment instructions and provided and sent home with the patient as shown in Figure 7 and 8, respectively.
 |
 |
| Figure 7: LipiFlow® pre-treatment instructions. | Figure 8: LipiFlow® post-treatment instructions. |
The quality of meibomian gland oils can be improved with daily fish oil supplementation or consumption of a diet that is high in omega-3 fatty acids. In more symptomatic cases, the chronic use of systemic tetracycline or macrolide antibiotics often has a dramatic effect on reducing the viscosity of inspissated meibomian oils. It is important that patients (and their primary care providers) understand that these medications are being used for their oil-altering effects, not their antibiotic properties, and that the daily intake of a probiotic tablet or the daily consumption of yogurt is recommended to minimize undue disruption of the gastrointestinal flora. In patients without rosacea, we typically use azithromycin 250 mg daily for 3 weeks, after which we reduce it to 3 times weekly (usually on Monday, Wednesday, and Friday). For patients with rosacea, we begin with minocycline or doxycycline 100 mg daily for 3 weeks and then reduce it to 3 times weekly. We continue the maintenance dose indefinitely if the patient is benefiting from therapy and there are no significant side effects, such as an upset stomach.
Ocular surface desiccation can be minimized with environmental manipulation, blink exercises, control of atopy, lipid layer supplementation, aqueous layer supplementation (if deficient), and surgical correction of eyelid abnormalities, such as lid laxity or orbicularis weakness or paralysis. Environmental manipulations include the use of humidifiers, avoidance of direct exposure to fans, minimization of exposure to blowing wind with the use of spectacles, and avoidance of allergens for allergic patients. Some patients, especially those with sleep apnea, benefit from the use of sleep goggles or masks. Blink exercises, which consist of episodic and sustained tight squeezing of the orbicularis oculi muscle may be effective in increasing the ratio of complete blinks and commensurate improvement in oil expression.[28] Patients with atopy should be treated with systemic and topical mast cell stabilizers and antihistamines. Patients with ocular surface itching in the absence of an atopic diathesis can be managed with topical treatment alone. We prefer the use of ketotifen fumarate (Zaditor®) twice a day or olopatadine hydrochloride (Patanol®).
When excessive tear evaporation occurs in conjunction with adequate aqueous tear production, the use of conventional wetting drops provides little or no relief. The use of punctal plugs is also without therapeutic benefit and may be associated with worsening of symptoms if ocular surface inflammation is present and not being properly addressed. However, the use of over-the-counter lubricants with high lipid content, such as Systane ®Balance, can minimize destabilization of the tear film and surface drying during aggravating environmental conditions.[29] When present, it is imperative that associated ATD be addressed with tear supplementation (https://webeye.ophth.uiowa.edu/eyeforum/tutorials/Artificial-Tears.htm) and punctal occlusion, after surface inflammation has properly been addressed. Failure to adequately address ATD and associated increased tear osmolarity may lead to progression of MGD and anatomic deterioration.[30] This may be the mechanism for induction of the severe and very hard-to-treat evaporative dry eye syndrome that persists following the resolution of post-LASIK neurotrophic-related ATD.[31]
When present, ocular surface inflammation should be controlled to relieve subjective symptoms and to prevent further meibomian gland deterioration. The most dramatic example of inflammation-induced destruction of the meibomian glands is seen with graft-versus-host disease, where complete loss of normal gland architecture can occur in 12 months. In most untreated cases of evaporative dry eye not associated with graft-versus-host disease, cicatricial conjunctival disorders, or rosacea, adequate control of inflammation can be achieved with a short-term course of intermediate-strength topical corticosteroids followed by continuation of a lower maintenance dose. Typically, we use topical fluorometholone (FML®) drops twice daily, along with dexamethasone 0.1% ointment at bedtime for 3 weeks, after which we discontinue the bedtime ointment but continue the topical drops, if necessary, to maintain comfort. With more severe inflammation, topical prednisolone acetate 1.0%, or equivalent, is utilized in sufficient dosage to eliminate subjective symptoms and clinical evidence of progression. If inflammation related to ATD is also present, we add Restasis® twice a day and maintain it indefinitely.
Acute bacterial overgrowth can be managed very effectively on a short-term basis with the simple use of topical erythromycin or bacitracin ointment at bedtime. Long-term control can be achieved with lid hygiene. Cleaning the eyelid margins with diluted Johnson's Baby Shampoo is the traditional remedy for morning discharge and eyelid crusting. Unfortunately, this treatment often relegates the role of hot compresses and digital massage as the primary treatment for MGD. The time consumption and complexity of this regimen, as well as its amelioration of only the "anterior blepharitis" component of the MGD symptom constellation, have played major factors in patients' discouragement and abandonment of therapy. Today, we only recommend a comprehensive eyelid regimen as part of the entire therapeutic package, but prefer the use of less cumbersome commercially available lid-cleaning products, such as SteriLid®, OcuSOFT®, or Avenova®.
Putting it All Together: "The Full Court Press"
When a patient begins MGD therapy, it is imperative that he/she enthusiastically commits to compliance and has realistic prognostic expectations. The guiding therapeutic principle is to provide "full court press" intervention for every applicable pathophysiologic mechanism while stressing the paramount importance of reestablishing meibomian gland patency.
In our practice, all newly diagnosed cases are placed on a baseline regimen that includes hot compresses and digital massage, lid hygiene, oral fish oil supplements, and environmental modification. Therapy is also provided for any pathophysiologic abnormalities that are present in addition to meibomian gland obstruction, including poor quality oils, ocular surface inflammation, and bacterial overgrowth.
The VAMC order set that addresses every potential abnormality includes the following:
- Hot compresses and digital massage once or twice daily
- Lid hygiene every morning with commercially available products
- Oral fish oil supplements 1000 mg daily
- Oral doxycycline/minocycline 100 mg or azithromycin 250 mg daily for three weeks followed by Monday, Wednesday and Friday daily
- Environmental modification
- Blink exercises, multiple daily sessions
- Systane® Balance 4 times a day
- Zaditor® or Patanol® twice a day
- FML® 0.1% twice a day
- Restasis® twice a day
- TobraDex® ointment both eyes at bedtime
The program may be quite laborious depending upon the number of factors that need to be addressed at the outset. It is important to understand that progress may be very gradual, or even nonexistent, during the first few weeks but that considerable relief is achievable with persistence.
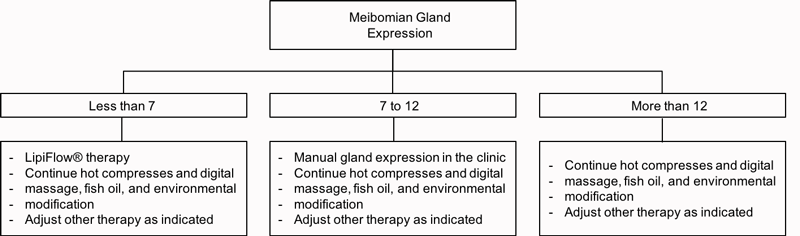
After 6 to 12 weeks, an MGE score-based algorithm is followed to address the progress or lack of progress with respect to re-establishment of gland patency as shown in figure 9.
Figure 9: MGE score-based treatment algorithm
Summary
EPIDEMIOLOGYMeibomian gland dysfunction (MGD) is a major cause of dry eye, occurs as part of normal aging, and is more prevalent in men and Asian populations. This entity is characterized by a quantitative oil deficiency due to obstruction-related hyposecretion and a qualitative oil deficiency due to production and secretion of poor quality oil. These MGD-related abnormalities lead to signs and symptoms that can be directly attributed to the instability of the outer lipid layer of the tear film with or without signs and symptoms of ocular surface inflammation and bacterial overgrowth |
SIGNSTear film instability
Ocular surface inflammation
Bacterial Overgrowth
|
SYMPTOMSTear film instability
Ocular surface inflammation
Bacterial overgrowth
|
MANAGEMENTRestore tear film stability
Control ocular surface inflammation
Reduce bacterial overgrowth
|
DIFFERENTIAL DIAGNOSIS
MGD-related Evaporative Dry Eye Syndrome
- Primary MGD
- Secondary MGD
- Acne Rosacea
- Atopy
- Chemical injury
- Chronic ocular surface inflammation
- Eyelid and orbicularis muscle disorders
- Medications (e.g., all-trans retinoic acid, antiandrogen medications)
- Mixed mechanism
ATD-related Dry Eye Syndrome
- Primary ATD
- Secondary ATD
- Autoimmune/collagen-vascular (including Sjögren's syndrome)
- Neurotrophic
- Medications
- Mixed mechanism
Combined Mechanism Dry Eye Syndrome
References
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea 2012;31(5):472-478.
- International Dry Eye Workshop. The definition and classification of dry eye disease: report of the International Dry Eye Workshop. Ocular Surf 2007;5:75-92.
- Nichol KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, Lemp MA, Sullivan DA. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci 2011;52(4):1922-1929.
- Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52(4):1994-2005.
- Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol 2013;7:1797-803.
- Greiner JV. Long-term (3-year) effects of a single thermal pulsation system treatment on meibomian gland function and dry eye symptoms. Eye Contact Lens 2016;42(2):99-107.
- Baumann A, Cochener B. Meibomian gland dysfunction: a comparative study of modern treatments. J Fr Ophtalmol 2014;37(4):303-12.
- Lane SS, DuBiner HB, Epstein RJ, Greiner JV, Hardten DR, Holland EJ, Lemp MA, McDonald JE 2nd, Silbert DI, Blackie CA, Stevens CA, Bedi R. A new system, LipiFlow®, for the treatment of meibomian gland dysfunction (MGD). Cornea 2012;31(4):396-404.
- Greiner JV. Long-term (12-month) improvement in meibomian gland function and reduced dry eye symptoms with a single thermal pulsation treatment. Clin Exp Ophthalmol 2013;41(6):524-530.
- Finis D, Ackermann P, Pischel N, Konig C, Hayajneh J, Borelli M, Schrader S, Geerling G. Evaluation of an automated thermodynamic treatment (LipiFlow®) system for meibomian gland dysfunction: a prospective, randomized, observer-masked trial. Ocul Surf 2014;12(2):146-54.
- Friedland BR et al. A novel thermodynamic treatment for meibomian gland dysfunction. Curr Eye Res 2011;36(2):79-87.
- Zhao Y, Veerapann A, Yeo S, Rooney DM, Acharya RU, Tan JH, Tong L. Clinical trial of thermal pulsation (LipiFlow) in meibomian gland dysfunction with pretreatment meibography. Collaborative Research Initiative for Meibomian Gland Dysfunction (CORIM). Eye Contact Lens 2016;42(6):339-346.
- Geerling G, Tauber J, Baudoin C, Goto E, Mutsumoto Y, O'Brien T, Rolando M, Tsuboto K, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2011;52(4):2050-64.
- Foulks GN, Nichols KK, Bron AJ, Holland EJ, McDonald MB, Nelson JD. Improving awareness, identification, and management of meibomian gland dysfunction. Ophthalmology 2012;119(10 Suppl):S1-12.
- Greiner JV, Glonek T, Korb DR. Volume of the human and rabbit meibomian gland system. Adv Exp Med Biol 1998;438:339-43.
- Nicolaides N et al. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci 1981;20(4):522-36.
- Knop E, Knop N, Miller T, Sullivan DA. The international workshop of meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci 2011;52(4):1938-78.
- Jalbert I. Diet, nutraceutricals and the tear film. Exp Eye Res 2013;1117:138-46.
- Truong S, Cole N, Stapleton F, Golebiowski B. Sex hormones and the dry eye. Clin Exp Optom 2014;97(4):324-36.
- Asiedu K. Rasch Analysis of the Standard Patient Evaluation of Eye Dryness Questionnaire. Eye Contact Lens 2017;43(6):394-398.
- Schiffman RM, Christianson MD, Jacobsen G, Hirsh JD, Reise BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118:615-621.
- Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea 2008;27:1142-1147.
- Korb DR, Greiner JV, Herman J. Comparison of fluorescein break-up time measurement reproducibility using standard fluorescein strips versus the dry eye test (DET) method. Cornea 2001;20:811-815.
- Finis D, Pischel N, Schrader S, Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for meibomian gland dysfunction. Cornea 2013;32(12):1549-53.
- King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci 2010;51(5):2418-2423.
- Stiff HA, Ricca AM, Bozung A, Goins KM. Corneal marginal ulcer: marginal keratitis with ulceration in a 45-year-old male. EyeRounds.org. posted March 14,2017. Accessed January 12, 2018; available from: https://eyerounds.org/cases/249-cornea-marginal-ulcer.htm
- Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX et al. TFOS DEWS II Management and Therapy Report. Ocul Surf 2017;15(3):575-628
- Korb DR, Baron DF, Herman JP, Finnemore VM, Exford JM, Hermosa JL, Leahy CD, Glonek T, Greiner JV. Tear film lipid layer thickness as a function of blinking. Cornea 1004;13(4):354-359.
- Aguilar AJ, Marquez MI, Albera PA, Tredicce JL, Berra A. Effects of Systane® Balance on noninvasive tear film break-up time in patients with lipid-deficient dry eye. Clin Ophthalmol 2014;8:2365-2372.
- Fenga C, Aragona P, Di Nola C, Spinella R. Comparison of ocular surface disease index and tear osmolarity as markers of ocular surface dysfunction in video terminal display workers. Am J Ophthalmol 2014;158(1):41-48.
- Dohlman CH, Lai EC, Ciralsky JB. Dry eye disease after refractive surgery. Int Ophthalmol Clin 2016;56(2):101-110.
Citing this article
Quist TS, Wagoner MD. Current Concepts in the Diagnosis and Management of Meibomian Gland Dysfunction-Related Evaporative Dry Eye Syndrome. EyeRounds.org. posted March 13, 2018; Available from: https://eyerounds.org/tutorials/Meibomian-gland-dysfunction-related-evap-dry-eye-syndrome/