
The University of Iowa
Department of Ophthalmology and Visual Sciences
Posted June 24, 2019
Photopsias (i.e., flashes) are a common presenting symptoms in the ophthalmology clinic. Although the majority of photopsias are retinal in origin [1], characterizing the appearance, onset, and associated features is critical in determining the cause of these prominent yet diverse descriptions of "flashing lights." The following tutorial discusses the various sources of photopsia: vitreoretinal traction, ocular migraines, age-related macular degeneration, diabetes, cerebral blood flow, visual hallucinations, cancer associated retinopathy, entoptic phenomenon, phosphenes, and lens associated dysphotopsias.
A posterior vitreous detachment (PVD) is a common cause of floaters and photopsias in the general population, accounting for approximately 40% of patients presenting with these symptoms [1]. Floaters (Figure 1) are typically due to cells or debris floating in the vitreous that cast shadows onto the retina. Patients also often describe seeing a large opaque floater as the vitreous separates from around the optic nerve head [2]. On clinical examination, this vitreous separation from the circular optic nerve can be seen as a Weiss ring.
Photopsias occur as the vitreous pulls on the retina. The tension from the vitreous on the retina causes retinal cells to fire and leads to the perception of flashes of light. These flashes typically last less than one second and are described as a lightning streak or a camera flash in the periphery. The shape of the lightning streak is usually curvilinear due to the edge of the vitreoretinal traction. Photopsias can occur unilaterally or bilaterally, but bilateral flashes typically occur at different times in each eye.
Retinal tears can also cause floaters and flashes of light in the periphery [1, 2]. Retinal tears due to traction from trauma or PVD are typically horseshoe shaped, and, if large enough, the vitreous enters the subretinal space causing a rhegmatogenous retinal detachment (RRD) [3]. Surgical intervention is required for RRDs, which are associated with persistent and progressive decrease in vision that patients typically described as a curtain or veil in their visual field. Differentiating between an acute PVD and retinal tears can be difficult based on history alone. Hollands, et al. found that 14% of patients presenting with floaters and/or flashes and a diagnosis of PVD also have a retinal tear; however, if there is no subjective decline in visual acuity, this risk decreases to 8.9% [4]. Conversely, if the patient reports a subjective decrease in visual acuity or a vitreous hemorrhage is seen, then the risk of a tear increases to 45% and 62%, respectively. If vitreous pigment (i.e., Shafer's sign) is noted, then the risk of a retinal tear is as high as 88% [4]. In patients diagnosed with a PVD without a retinal tear, 3.4% had a retinal tear within six weeks of their initial presentation [4]. Thus, all patients should have a repeat dilated fundus examination within 4-6 weeks after initial presentation.

Migraine is a recurrent, frequently unilateral, headache syndrome often with a prodrome of bilateral positive visual phenomena [2]. A "classic migraine" is described by having this prodrome (i.e., aura) lasting about 15 to 30 minutes and followed by a headache and related symptoms that can persist for hours [5]. Although the visual phenomenon typically occurs bilaterally, the photopsias may appear larger in one eye than the other [1]. Visual symptoms can occur with every migraine headache an individual has or may only happen once [1]. Migraine and auras are not fully understood, and there is much debate around their underlying mechanisms. A leading theory is that migraines are caused by disturbances in cerebral blood flow and a wave of depressed neuronal activity moves slowly across the brain; this process usually starts in the occipital lobe and spreads anteriorly. A migraine aura likely is a result of the initial wave of high neuronal activity related to the previously described spreading depression followed by an inhibition of activity [5]. Auras can manifest as small bright lights, blind spots, static/foggy vision, and/or complex visual disturbances. Typically, auras begin prior to the headache as a central crescent-shaped scintillating scotoma that expands outwards and is surrounded by flashes or zigzags of light [1, 6]. In a retinal migraine, a patient experiences decrease in vision or complete blindness in one eye without a scintillating scotoma; this is due to vasospasm of the retinal circulation or ophthalmic artery. Vision in a retinal migraine promptly returns to normal. A very uncommon form of migraine is an ophthalmoplegic migraine, which can cause a temporary paralysis of one of the three cranial nerves involved in ocular motion (CN III, CN IV, and CN VI) but is not associated with photopsias [7].


Non-exudative (i.e., dry) age-related macular degeneration (AMD) causes gradual bilateral central visual loss without related pain. Patients will report decreased central vision and metamorphopsia but do not usually have photopsias associated with dry AMD. Exudative (i.e., neovascular or wet) AMD, however, is another common cause of photopsias and the second most common cause in one reported case series [1]. Approximately 50% of individuals with exudative AMD report experiencing repeated centrally located flashes that last for several seconds to a few minutes. These flashes are typically described as flickers, pulsations, sparkling lights, snake-like lights, spinning lights, pinwheels, or circles. These lights are most commonly white in color, but people have reported seeing blue, silver, gold, or multiple colored lights [1]. The likelihood of reported photopsias increases as the neovascular membranes increases in area. Unlike the photopsias of PVD, which are stimulated from the inner retina-vitreous interface, the photopsias from exudative AMD occur from accumulating fluid stimulating the outer retina layers [1]. Differentiating between the two may sometimes be difficult prior to exam; however, central flashes are far more common with AMD and peripheral flashes are more common with PVD [1].
Diabetes can cause a multitude of visual changes. Progression of the disease can lead to proliferative or non-proliferative diabetic retinopathy. Diabetic patients with tight control of their blood glucose levels can significantly decrease their risk of developing diabetic retinopathy [8-10]. However, the majority of patients will develop diabetic retinopathy after 15 years with the disease [11, 12]. Non-proliferative diabetic retinopathy is characterized by microaneurysms, dot-and-blot hemorrhages, hard exudates, cotton-wool spots, and macular edema. Patients with macular edema will commonly present with a decrease in visual acuity [13]. However, most patients will remain asymptomatic until they reach the proliferative phase. Proliferative diabetic retinopathy occurs when the prolonged ischemia to the retina triggers new vessel and fibrous tissue formation. The new fibrous growth forms a contracted scar at the vitreoretinal interface and can cause photopsias as the tissue contracts. This contraction can lead to tractional retinal detachments (TRD) with a potential vitreous hemorrhage due to these new vessels being fragile [9]. Patients with TRDs may report floaters, photopsias, and/or a curtain over their visual field that is similar to rhegmatogenous retinal detachments. The complications of proliferative diabetic retinopathy can result in permanent vision loss. In a small study, patients with hypoglycemia who were insulin-dependent diabetics reported bilateral photopsias that ceased when their glucose returned to normal levels. They occurred in either light or dark settings and were described as white flickers or circles [1].
Vertebral basilar insufficiency causes reduced blood flow to the posterior aspect of the brain and is associated with aging. This decreased blood flow causes ischemia to this area and leads to bilateral photopsias, which are described as broken flashes lasting seconds to minutes [1, 14]. In addition, vertebral basilar insufficiency is associated with vertigo, dizziness, diplopia, blindness, weakness, and ataxia [14]. Patients may have similar flashes of light and fogging of vision, similar to a visual migraine; however, these symptoms do not continue for as long and do not occur before a headache [1, 3].
Release hallucinations are characterized as visual hallucinations resulting from damage to the visual pathway, unilaterally or bilaterally, in individuals with intact cognition [13]. Patients describe seeing multi-colored shapes, grids, faces, people, and flowers lasting from seconds to minutes [1]. The underlying mechanism is poorly understood. The most widely accepted theory is the sensory deprivation theory, which states that the loss of visual stimuli to the visual cortex increases the excitability of neurons. This increased neuronal activity leads to random firing of the neurons with little to no stimuli, hence the name release hallucinations [15]. This is supported by the high prevalence in individuals with poorer visual acuity and after postoperative eye patching [16, 17]. The prevalence of Charles Bonnet Syndrome increases in individuals with poorer visual acuity bilaterally [13, 15].
Cancer-associated retinopathy is rare autoimmune disease where the body develops autoantibodies to retinal antigens [18]. Recoverin and a-enolase are the most common retinal antigens to which autoantibodies develop [19]. These antibodies typically develop in the presence of malignancy, most frequently small cell lung cancer [18-20]. Patients who develop this disease often present with a decrease in visual acuity secondary to photoreceptor dysfunction. Individuals may experience photosensitivity, increased glare after light exposure, decrease in color vision, and/or central scotomas and sausage-shaped arcuate (Bjerrum) scotomas due to cone dysfunction. Night blindness, peripheral ring scotomas, or a significant decline in peripheral vision can be seen with rod dysfunction [18, 20]. Photopsias are also seen with cancer-associated retinopathy approximately 7-15% of the time. They are described as flickering or shimmering lights and are thought to be caused by retinal degeneration. One study found that visual symptoms may proceed a cancer diagnosis [21].
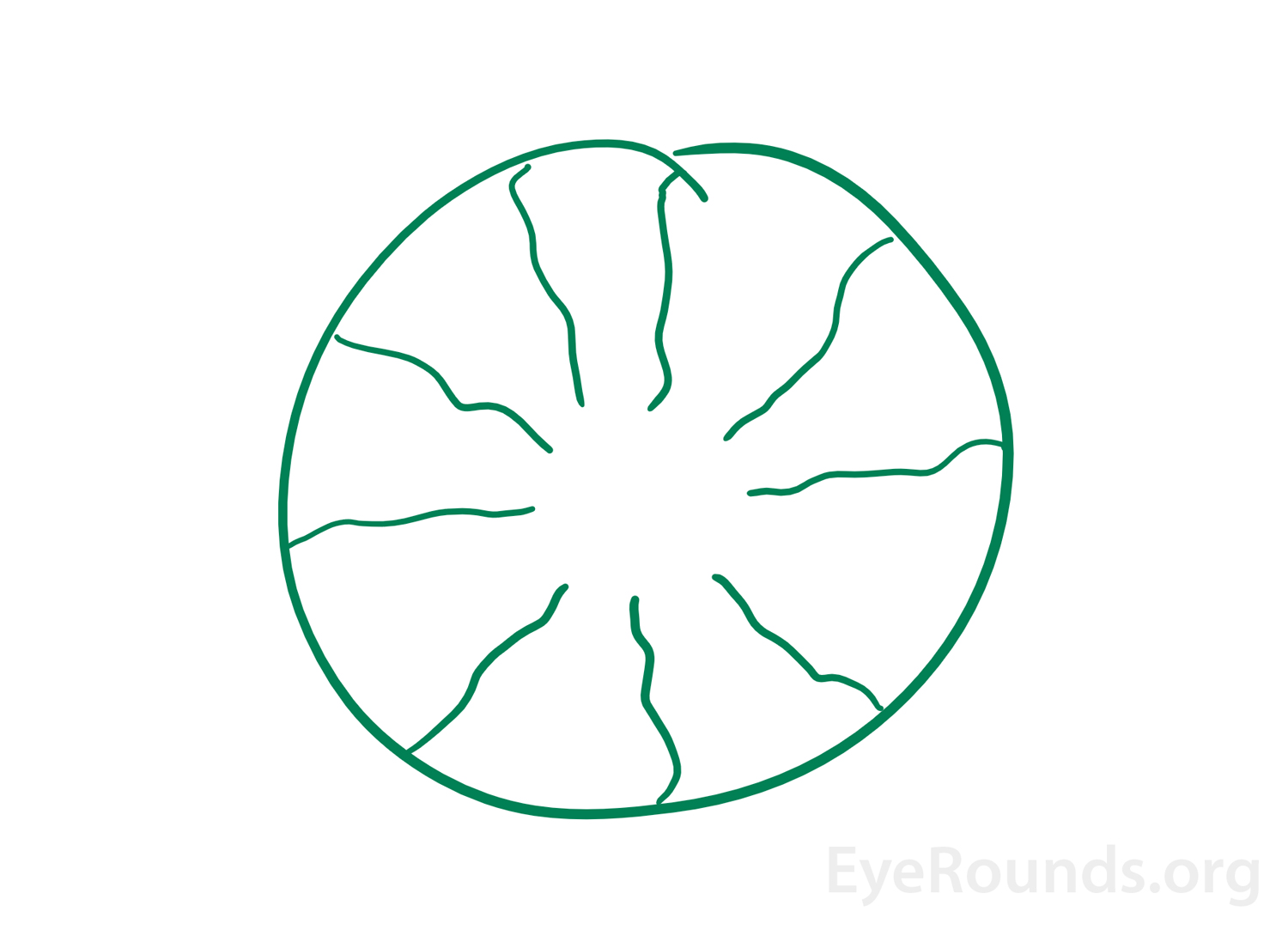
When individuals perceive an image that originates within their own eyes this is known as an entoptic image (Figure 3). This phenomenon can arise from shining a penlight in contact with an individual's closed eyelids then moving the penlight [22]. The images that appear with this are described as a branching pattern of lines due to the shadow cast by retinal vessels on to other areas of the retina [23, 24]. Sometimes, the image may flash and pulsate with the heartbeat and will remain for a few seconds as the light source continues to move [1]. In the past, this phenomenon was used as an alternate test of visual acuity. If an individual was able to see the branching pattern in one eye but not the other, the eye that did not see the image was determined to have poorer macular function [24].
Phosphenes are a positive photopsia that are seen without a light source [25]. They are described as flashes of light, bars/spots of light, or colored spots. These can be elicited by rubbing the eyes, coughing, head trauma, or from other pathological causes. Production of phosphenes by these mechanisms is thought to be due to excitation the photoreceptors in the retina through mechanical pressure [26]. The other underlying mechanisms vary depending on the pathology seen within the eye. Retinal traction, retinal detachments, cancer-associated retinopathy, and release hallucination are all pathological causes of phosphenes and occur as discussed above. Phosphenes are also experienced with substance intoxications or irradiation to the eye [27].
Positive and negative dysphotopsias are common occurrences following cataract extraction with placement of an intraocular lens. The typical description given of positive dysphotopsias are flashes of light, glare, or halos present in the periphery [28]. These occur when the eyes are open and vary in different settings of light, most commonly occurring when an individual enters a lighted room from the dark when the pupils are dilated. These are in contrast to photopsias due to vitreous traction, which are typically elicited in the dark and triggered via movements of the eye. Unlike retinal detachments, positive dysphotopsias are not persistent and do not increase in size [29]. The underlying mechanism of positive dysphotopsias is the result of aberrant reflections of light off the edge of the intraocular lens [28].
In contrast, negative dysphotopsias are commonly described as arcs, or crescent-shaped, shadows in the periphery [28]. The underlying mechanism of negative dysphotopsias is due to the fraction of light that reflects away from the eye causing a small area in which light does not reach the retina [29]. Negative dysphotopsias commonly resolve when the patient is dilated. Both positive and negative dysphotopsias are more common with small sharp-edged IOLs [28-30]. Multifocal IOLs are also associated with an increase in glare and photopsias when compared to monofocal IOLs [31]. These symptoms are most common in the early post-operative period but typically subside as the capsule undergoes fibrosis [28]. Although relatively infrequent, some patients may find these distracting enough to require repositioning or secondary placement of the intraocular lens.
Morrow N, Chung AT, Wall M. Photopsias. EyeRounds.org. June 24, 2019; Available from https://EyeRounds.org/tutorials/photopsias/index.htm